Tests
1st tests to order
real-time reverse transcription polymerase chain reaction (RT-PCR)
Test
Order an RT-PCR for SARS-CoV-2 in patients with suspected infection, whenever possible.[439] Commonly used assays are expected to be able to detect SARS-CoV-2 variants.[495] Genomic sequencing may be performed to differentiate between variants.
Testing strategies vary widely between countries and you should consult your local guidance. For more detailed information on who to test and choice of test see Diagnosis recommendations.
The optimal specimen for testing depends on the clinical presentation and the time since symptom onset. The World Health Organization (WHO) recommends upper respiratory specimens (nasopharyngeal and/or oropharyngeal swabs) for early-stage infections, especially asymptomatic or mild cases, and lower respiratory specimens (sputum and/or endotracheal aspirate or bronchoalveolar lavage in patients with more severe respiratory disease) for later-stage infections or patients in whom there is a strong suspicion for infection and their upper respiratory tract specimen test was negative. Other specimens (e.g., nasal mid-turbinate swab, anterior nares swab, nasopharyngeal/nasal wash/aspirate, saliva, fecal) may be recommended in some circumstances; consult local guidance.[439][496][614]
A positive RT-PCR result confirms SARS-CoV-2 infection (in the context of the limitations associated with RT-PCR testing). If the result is negative, and there is still a clinical suspicion of infection (e.g., an epidemiologic link, typical x-ray findings, absence of another etiology), resample the patient and repeat the test. A positive result confirms infection. If the second test is negative, consider serologic testing (see below).[439] The pooled sensitivity has been estimated to be 87.8%, with the specificity estimated to be in the range of 87.7% to 100%.[502]
Molecular testing is an aid to diagnosis only and results should be interpreted with caution. For detailed information on testing limitations and evidence, see Diagnosis recommendations.
Single-test multiplex assays to diagnose and differentiate between infection caused by influenza A, influenza B, respiratory syncytial virus, and SARS-CoV-2 are available in some countries, including over-the-counter at-home tests.
Collect nasopharyngeal swabs to rule out influenza and other respiratory infections according to local guidance.[444] It is important to note that coinfections can occur, and a positive test for a non-COVID-19 pathogen does not rule out COVID-19.
Result
positive for SARS-CoV-2 viral RNA; may be positive for influenza A and B viruses and other respiratory pathogens
rapid antigen test
Test
Rapid antigen tests may be used in some settings as an alternative to (or in addition to) RT-PCR. Testing strategies vary widely between countries and you should consult your local guidance.[440][442][503][615] For more detailed information on who to test and choice of test, see Diagnosis recommendations.
While antigen tests are substantially less sensitive than RT-PCR, particularly in asymptomatic people, they offer the possibility of rapid, inexpensive, and early detection of the most infectious cases in appropriate settings.[440] Results are usually available in less than 30 minutes.
A Cochrane review found that rapid antigen tests vary in sensitivity. Sensitivity was higher in the first week after symptom onset in symptomatic people (80.9%), compared with the second week of symptoms (53.8%). Sensitivity was higher in symptomatic people (73%), compared with asymptomatic people (54.7%). Sensitivity also varied between brands of tests.[538]
Rapid antigen tests are an aid to diagnosis only and results should be interpreted with caution. For detailed information on testing limitations and evidence, see Diagnosis recommendations.
Rapid, lateral flow antigen tests for home use are available in some countries. Laboratory-based (nonrapid) antigen tests are also available in some countries.
BMJ: interpreting a lateral flow SARS-CoV-2 antigen test Opens in new window
Resultado
positive for SARS-CoV-2 virus antigen
pulse oximetry
Exame
Pulse oximetry may reveal hypoxia/hypoxemia (i.e., oxygen saturation <94%, or <88% in the presence of chronic lung disease).[474]
The World Health Organization (WHO) defines severe disease as SpO₂ <90%.[444] In the UK, a reading of <92% is one defining feature of severe disease that requires urgent hospital admission, while a reading of 93% to 94% may indicate moderate disease.[475] In the US, the Infectious Diseases Society of America (IDSA) defines severe disease as SpO₂ <94%.[401]
Clinicians should be aware that patients with COVID-19 can develop "silent hypoxia": their oxygen saturations can drop to low levels and precipitate acute respiratory failure without the presence of obvious symptoms of respiratory distress.[476]
Pulse oximeters may exhibit suboptimal accuracy in certain populations, especially in those who have darker skin.[477] The Food and Drug Administration (FDA) has warned that multiple factors can affect the accuracy of a pulse oximeter reading (e.g., poor circulation, skin pigmentation, skin thickness, skin temperature, current tobacco use, use of fingernail polish). The FDA recommends considering accuracy limitations when using a pulse oximeter to assist in diagnosis and treatment decisions, and to use trends in readings over time rather than absolute cut-offs if possible.[478]
Pulse oximeters can be used at home to detect hypoxia in patients with mild to moderate disease. Evidence suggests that patients who may benefit most from monitoring are those who are symptomatic and are either over age 65 years, or are under age 65 years and are extremely clinically vulnerable to COVID-19.[475] Home pulse oximetry requires clinical support (e.g., regular phone contact from a health professional).
Результат
may show low oxygen saturation (cut-off depends on local guidelines)
ABG
.
Order in patients with severe illness as indicated to detect hypercarbia or acidosis.
Recommended in patients with respiratory distress and cyanosis who have low oxygen saturation.
Результат
may show low partial oxygen pressure
CBC
.
Order in patients with severe illness.
Lymphopenia, leukocytosis, thrombocytopenia, decreased eosinophils, decreased hemoglobin, and high neutrophil-to-lymphocyte ratio are significantly associated with severe disease, and may be useful for predicting disease progression. Severe cases are more likely to present with lymphopenia and thrombocytopenia, but not leukopenia.[616]
Anemia is common and may be associated with a higher risk of mortality.[617]
Elevated red blood cell distribution width (at admission and increasing during hospitalization) has been associated with a significantly increased risk of mortality in hospitalized patients.[618]
Absolute counts of major lymphocyte subsets, particularly CD4+ and CD8+ T-cell counts, are significantly decreased in patients with severe disease.[619]
Late-phase thrombocytopenia (i.e., occurring 3 weeks or more after symptom onset) has been reported but is uncommon.[620]
Результат
lymphopenia; leukocytosis; leukopenia; thrombocytopenia; decreased eosinophils; decreased hemoglobin
comprehensive metabolic panel
.
Order in patients with severe illness.
Elevated liver enzymes, total bilirubin, creatinine, and blood urea nitrogen, and hypoalbuminemia are significantly associated with severe disease, and may be useful for predicting disease progression.[616]
Hypokalemia has been reported in 54% of patients.[621] Hypocalcemia has been reported and is associated with poor outcomes.[622] Hyponatremia has been reported in 24% of patients, and is associated with poor outcomes.[623] Other electrolyte derangements may be present.
Result
elevated liver enzymes; elevated total bilirubin; renal impairment; hypoalbuminemia; electrolyte derangements
blood glucose level
Test
Order in patients with severe illness.
Fasting hyperglycemia independently predicts poor prognosis and is associated with an increased risk of mortality, regardless of whether or not the patient has diabetes.[624][625]
Hypoglycemia has also been associated with increased mortality in a retrospective cohort study.[626]
Infection is associated with an increased risk of new-onset diabetes. Therefore, monitoring for hyperglycemia during admission should be considered.[627]
Result
variable
coagulation screen
Test
Order in patients with severe illness.
Elevated D-dimer, elevated fibrinogen (and fibrin degradation product), and prolonged prothrombin time are significantly associated with severe disease, and may be useful for predicting disease progression.[616][628]
The risk of severe disease and mortality is 2-fold and 4-fold higher, respectively, in patients with elevated D-dimer levels.[629] Patients with very high D-dimer levels have an increased risk of thrombosis.[630][631]
Prolonged international normalized ratio (INR) values have been associated with more severe disease and mortality.[632]
Von Willebrand factor markers may be increased, especially in patients with critical disease, and may have prognostic value.[633]
Result
elevated D-dimer; prolonged prothrombin time; elevated fibrinogen; prolonged INR
cardiac biomarkers
Test
Order in patients with severe illness.
Elevated creatine kinase-myocardial band (CK-MB), B-type natriuretic peptide (BNP), N-terminal proBNP (NT-proBNP), and troponin are associated with severe disease and mortality, and may be useful for predicting disease progression or survival.[634]
CK-MB has been found to be elevated in mild disease in children. The significance of this is unknown.[486]
Result
may be elevated
serum C-reactive protein
Test
Order in patients with severe illness.
Elevated C-reactive protein is significantly associated with severe disease, and may be useful for predicting disease progression.[616][635]
Patients with elevated C-reactive protein at the time of initial presentation were more likely to have acute kidney injury, venous thromboembolism, critical illness, and in-hospital mortality during their hospital stay compared with patients with lower levels.[636]
Result
may be elevated
serum erythrocyte sedimentation rate
Result
may be elevated
serum lactate dehydrogenase
Test
Order in patients with severe illness.
Elevated serum lactate dehydrogenase is significantly associated with severe disease, and may be useful for predicting disease progression.[616]
Result
may be elevated
serum procalcitonin
Test
Order in patients with severe illness.
Elevated serum procalcitonin is significantly associated with severe disease, and may be useful for predicting disease progression and mortality.[616][637]
Elevated serum procalcitonin may be more common in children.[638]
May be elevated in patients with secondary bacterial infection.[29][30]
There is insufficient evidence to recommend routine procalcitonin testing to guide decisions about the use of antibiotics. However, it may be helpful in identifying whether there is a bacterial infection, although the most appropriate procalcitonin threshold is uncertain.[405] Do not perform procalcitonin testing without an established, evidence-based protocol.[639]
Result
may be elevated
serum ferritin level
blood and sputum cultures
chest x-ray
Test
Order in all patients who are seriously ill (e.g., SpO₂ <94% or National Early Warning Score 2 [NEWS2] score ≥3) or those who are stable but a chest x-ray is clinically indicated (e.g., suspected pneumonia).[443]
Approximately 74% of patients have an abnormal chest x-ray at the time of diagnosis. The most common abnormalities are ground-glass opacity (29%) and consolidation (28%). Distribution is generally bilateral, peripheral, and basal zone predominant. Pneumothorax and pleural effusions are rare. There is no single feature on chest x-ray that is diagnostic for COVID-19.[546]
Chest x‐ray is moderately sensitive and moderately specific for the diagnosis of COVID‐19. Pooled results found that chest x‐ray correctly diagnosed COVID‐19 in 73% of people who had the disease. However, it incorrectly identified COVID‐19 in 27% of people who did not have the disease.[547]
Although chest x-ray appears to have a lower sensitivity compared with chest CT, it has the advantages of being less resource-intensive, associated with lower radiation doses, easier to repeat sequentially, and portable.[544]
Resultado
ground-glass opacity; consolidation
Investigações a serem consideradas
computed tomography (CT) chest
Exame
Consider a CT scan of the chest. Consult local guidance on whether to perform a CT scan.
The British Society of Thoracic Imaging (BSTI) recommends CT imaging in patients with clinically suspected COVID-19 who are seriously ill if chest x-ray is uncertain or normal. BSTI: radiology decision tool for suspected COVID-19 Opens in new window Some institutions in the UK recommend a more pragmatic approach for patients with high clinical suspicion of COVID-19, with chest CT recommended only after two indeterminate or normal chest x-rays in combination with a negative RT-PCR test.[549]
The American College of Radiology recommends reserving CT for hospitalized, symptomatic patients with specific clinical indications for CT, and emphasizes that a normal chest CT does not mean that a patient does not have COVID-19 and that an abnormal chest CT is not specific for COVID-19 diagnosis.[550]
Evidence of pneumonia on CT may precede a positive RT-PCR result for SARS-CoV-2 in some patients.[554] Some patients may present with a normal chest finding despite a positive RT-PCR.[555] Results of RT-PCR testing may be false-negative, so patients with typical CT findings should have repeat RT-PCR testing to confirm the diagnosis.[556] CT imaging abnormalities may be present in asymptomatic patients. The pooled estimate of the rate of positive chest CT findings in asymptomatic cases was 47.6% (mainly ground-glass opacity).[557]
Abnormal chest CT findings have been reported in up to 97% of hospitalized patients.[559] The most common findings are ground-glass opacity, either in isolation or coexisting with other findings such as consolidation, interlobular septal thickening, or crazy-paving pattern. The most common distribution pattern is bilateral, peripheral/subpleural, posterior distribution of the opacities, with a lower lobe predominance. Extensive/multilobar involvement with consolidations is more common in older patients and those with severe disease. Atypical features include pulmonary vascular enlargement, adjacent pleural thickening, air bronchograms, subpleural lines, bronchus distortion, bronchiectasis, vacuolar retraction sign, and halo sign. Pleural effusion, pericardial effusion, cavitation, pneumothorax, and mediastinal lymphadenopathy have been reported rarely.[560] Ground-glass opacity has the highest diagnostic performance for COVID-19 pneumonia, followed by ground-glass opacity plus consolidation, and consolidation only.[561]
Patients with Omicron variants of SARS-CoV-2 were less likely to be CT-positive compared with those infected with non-Omicron variants. Patients with Omicron variants typically had lesions located in the center of the lung or in a single lobe. They were less likely to have bilateral lesions, crazy-paving pattern, linear opacity, and vascular enlargement, but were more likely to have bronchial wall thickening. The distribution of lesions was more extensive (bilateral and multilobe involvement) in patients with non-Omicron variants.[558]
Pregnant women appear to present more commonly with more advanced CT findings compared with the general adult population; however, results are similar to those in the general adult population.[564]
Children frequently have normal or mild CT chest findings. The most common signs in children are patchy ground-glass opacity, nonspecific patchy shadows, areas of consolidation, infected nodules, and a halo sign. Abnormalities are more common in multiple lobes and are predominantly bilateral. Pleural effusion is rare.[565][642] Ground-glass opacity and peribronchial thickening were the most prevalent findings in infants younger than 1 year of age.[566] Accuracy appears to be lower among children; however, there are limited data in this population.[548]
Chest CT generally shows an increase in the size, number, and density of ground-glass opacities in the early follow-up period, with a progression to mixed areas of ground-glass opacities, consolidations, and crazy paving peaking at day 10 to 11, before gradually resolving or persisting as patchy fibrosis.[560]
Chest CT is moderately sensitive (70%) and highly specific (90%) for the diagnosis of COVID‐19.[551] Pooled results found that chest CT correctly diagnosed COVID‐19 in 87% of people who had the disease. However, it incorrectly identified COVID‐19 in 21% of people who did not have the disease. Therefore, chest CT may have more utility for excluding COVID‐19 than for differentiating it from other causes of respiratory illness.[547] The positive predictive value was low (1.5% to 30.7%) in low-prevalence regions, and the negative predictive value ranged from 95.4% to 99.8% in one meta-analysis. Pooled sensitivity and specificity were 94% to 96% and 37%, respectively.[552][553] The simultaneous presence of ground-glass opacity and other features of viral pneumonia had optimum performance in the detection of COVID-19 (sensitivity 90% and specificity 89%).[562]
[Figure caption and citation for the preceding image starts]: Transverse CT scans from a 32-year-old man, showing ground-glass opacity and consolidation of lower lobe of right lung near the pleura on day 1 after symptom onset (top panel), and bilateral ground-glass opacity and consolidation on day 7 after symptom onsetXu XW et al. BMJ. 2020;368:m606 [Citation ends].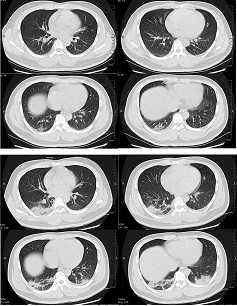
Result
ground-glass opacity in isolation or coexisting with other findings (e.g., consolidation, interlobular septal thickening, crazy-paving pattern); bilateral, peripheral/subpleural, posterior distribution with a lower lobe predominance
lung ultrasound
Test
Used as a diagnostic tool in some centers as an alternative to chest x-ray and chest CT. Although there is only very low-certainty evidence supporting its diagnostic accuracy, it might be helpful as a supplemental or alternate imaging modality.[544]
Ultrasound is sensitive but not specific for the diagnosis of COVID‐19. Pooled results found that lung ultrasound correctly diagnosed COVID‐19 in 87% of people with the disease. However, it incorrectly diagnosed COVID‐19 in 24% of people who did not have the disease. Therefore, ultrasound may have more utility for excluding COVID‐19 than for differentiating it from other causes of respiratory illness.[547] It may also be useful for triage in the emergency department.[643]
B-lines (confluent or separated and usually at least 3) and pleural abnormalities, with a bilateral distribution, are the most frequent findings in COVID-19. Other findings include consolidations, pleural effusion, air bronchogram, and pneumothorax.[568] While these findings are not specific for COVID-19, they increase the likelihood of disease in the context of a characteristic clinical presentation.
Has the advantages of portability, bedside evaluation, reduced healthcare worker exposure, easier sterilization process, absence of ionizing radiation exposure, repeatability during follow-up, may be more readily available in resource-limited settings, and is safe in pregnant women and children. However, it also has some limitations (e.g., it is unable to discern chronicity of a lesion) and other imaging modalities may be required.[569][570][571]
Possible roles include: reducing nosocomial transmission; monitoring progress of patients; and a possible role in subpopulations who are vulnerable but are not suitable for CT (e.g., pregnant women).[572] Lung ultrasound score may play a role in prognosis.[573]
BSTI: lung ultrasound (LUS) for COVID-19 patients in critical care areas Opens in new window
Result
B-lines; pleural abnormalities
serology
Test
Cannot be used as a standalone diagnostic for acute infections, and should not be used to establish the presence or absence of acute infection; however, may be useful in various settings.[439][507]
Antibody responses to SARS-CoV-2 typically occur during the first 1 to 3 weeks of illness, with the seroconversion time of IgG antibodies often being earlier than that of IgM antibodies.[505][506]
The World Health Organization (WHO) recommends collecting a paired serum sample, one specimen in the acute phase and one in the convalescent phase 2 to 4 weeks later, in patients where infection is strongly suspected and the RT-PCR result is negative. Seroconversion or a rise in antibody titers in paired sera help to confirm whether the infection is recent and/or acute. If the initial sample tests positive, this could be due to a past infection that is not related to the current illness. Seroconversion may be faster and more robust in patients with severe disease compared with those with mild disease or asymptomatic infection.[439]
The Infectious Diseases Society of America (IDSA) recommends against using serologic testing to diagnose infection during the first 2 weeks following symptom onset or to provide evidence of infection in symptomatic patients with a high clinical suspicion and repeatedly negative molecular tests. Serologic testing may be used to assist with the diagnosis of multisystem inflammatory syndrome in children or when evidence of previous infection is desired.[507]
A Cochrane review found that antibody tests could be a useful tool for patients in whom molecular- or antigen-based tests have failed to detect SARS-CoV-2 virus, including in those with ongoing symptoms of acute infection (from week 3 onwards) or those presenting with post-acute sequelae. Test sensitivity is too low during the first week since the onset of symptoms.[543]
The estimated sensitivity of antibody tests ranged from 18.4% to 96.1% (the lowest reported sensitivity was from a point-of-care test, although a sensitivity <50% was reported for one laboratory test), and specificity ranged from 88.9% to 100%. Estimates of diagnostic accuracy need to be interpreted with caution in the absence of a definitive reference standard to diagnose or rule out COVID-19.[502]
For detailed information on testing limitations and evidence, see Diagnosis recommendations.
While rapid antibody detection kits have been approved for the qualitative detection of SARS-CoV-2 IgG/IgM antibodies in serum, plasma, or whole blood, the WHO does not recommend the use of these tests outside of research settings as they have not been validated as yet.[508] Evidence does not support their use.[509]
BMJ practice pointer: testing for SARS-CoV-2 antibodies Opens in new window
Result
positive for SARS-CoV-2 virus antibodies; seroconversion or a rise in antibody titers in paired sera
Use of this content is subject to our disclaimer
