Epidemiology
The World Health Organization (WHO) was informed of cases of pneumonia of unknown microbial etiology associated with Wuhan City, Hubei Province, China on 31 December 2019. The WHO later announced that a novel coronavirus had been detected in samples taken from these patients. Since then, the epidemic escalated and rapidly spread around the world, with the WHO first declaring a public health emergency of international concern (PHEIC) on 30 January 2020, and then formally declaring it a pandemic on 11 March 2020. The WHO declared that COVID-19 no longer constitutes a PHEIC in May 2023.
Cases have been reported across all continents since the beginning of the pandemic, with over 777 million confirmed cases and over 7 million deaths reported globally.[17]
[Figure caption and citation for the preceding image starts]: Number of COVID-19 cases reported weekly by WHO Region, and global deaths, as of 2 February 2025World Health Organization [Citation ends].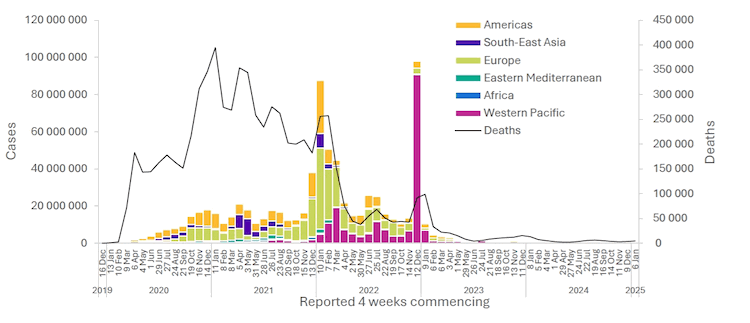
Updated case counts are available from the WHO and the Centers for Disease Control and Prevention (CDC):
Older people age ≥70 years and males are at increased risk for infection and severe disease.[18] Adolescents appear to have similar susceptibility to infection as adults, and children have a lower susceptibility. However, evidence is conflicting.[19][20] Unlike adults, children do not seem to be at higher risk for severe disease based on age or sex.[21] Variants may have spread more effectively and rapidly among young children compared with the wild-type virus, although hospitalization rates decreased.[22][23]
The incidence of infection in healthcare workers ranged from 0% to 49.6%, and the prevalence of seropositivity ranged from 1.6% to 31.6%. There was no association between age, sex, or healthcare worker role (i.e., nurse versus physician) and the risk for infection, based on moderate-certainty evidence. There was an association between black race or Hispanic ethnicity and increased risk for infection in healthcare workers compared with white race or non-Hispanic ethnicity, based on moderate-certainty evidence. There was an association between use of personal protective equipment and decreased risk for infection, based on moderate-certainty evidence.[24][25]
Risk factors
People who have been in contact with a probable or confirmed case are at increased risk of infection.
The World Health Organization (WHO) defines a contact as a person who has experienced any one of the following exposures: face-to-face contact with a probable or confirmed case within 3 feet (1 meter) and for at least 15 minutes; direct physical contact with a probable or confirmed case; direct care for a patient with probable or confirmed COVID-19 without using recommended personal protective equipment; or other situations as determined by local health authorities based on local risk assessments. Exposure must have occurred during the infectious period of the case. For symptomatic cases, this means 2 days before and 10 days after symptom onset of the case, plus at least 3 additional days without symptoms, for a minimum of 13 days total after symptom onset. For asymptomatic cases, this means 2 days before and 10 days after the date on which the sample that led to confirmation was taken.[124]
People who live or work in a location with a high risk of transmission are at increased risk of infection.
People residing or working in an area with a high risk of transmission (e.g., closed settings, humanitarian settings) and people working in a health setting (including within health facilities and households) are at higher risk of infection.[124]
Older people are at increased risk for infection and severe disease.[18]
The risk of hospitalization and death increases with age. An increased age-related risk of in-hospital mortality, case mortality, and hospitalization of 5.7%, 7.4%, and 3.4% per age year, respectively, has been observed, based on high-quality evidence. No increased risk was observed for intensive care unit admission and intubation by age year. There was no evidence of a specific age threshold at which the risk accelerates considerably.[125]
According to US data, the risk of death is 340 times higher in people ages 85 years and older compared with 18- to 29-year-olds.[126]
In the UK, data from a cross-sectional study indicated that people ages 40 to 64 years are at greatest risk of infection, followed by patients 75 years and older, and then people ages 65 to 74 years.[127] The highest mortality rate was observed in patients 80 years and older.[128]
In the US, patients ≥65 years accounted for 31% of all cases, 45% of hospitalizations, 53% of intensive care unit admissions, and 80% of deaths early in the pandemic, with the highest incidence of severe outcomes in patients ages ≥85 years.[129]
While age is an independent risk factor, the risk in older people is also partly related to the likelihood that older adults are more likely to have comorbidities. The higher prevalence of malnutrition in older patients may also contribute to poor outcomes.[130]
Males are at increased risk for infection and severe disease.[18]
A meta-analysis found that men have a higher risk for infection, hospitalization, disease severity, intensive care unit admission, and death.[131]
Various hypotheses have been proposed to explain the difference including androgen-driven pathogenesis (evidence is weak), immunologic protective effect of estrogen in females (conflicting evidence), testosterone deficiency-induced inflammatory storm (limited conflicting evidence), and inborn error of cytokine immunity (requires more investigation). The cause is likely multifactorial with overlapping features of various hypotheses.[132]
People who belong to racial/ethnic minority populations may be at increased risk of infection, severe disease, hospitalization, intensive care unit admission, and death to varying extents across ethnic groups.[133][134][135][136] However, studies are inconsistent, particularly in regards to the definitions of racial/ethnic minority groups and socioeconomic status.
In the UK, data indicate that South Asian, black, and mixed ethnicity populations have an increased risk for testing positive and of adverse outcomes (i.e., hospitalization, intensive care unit admission, death) compared with the white population, even after accounting for differences in sociodemographic, clinical, and household characteristics.[137][138][139] Race may play an important role in adverse outcomes in children as well as adults.[140]
In the US, American Indian or Alaskan Native, Latino, black, and Asian or Pacific Islander people were more likely than white people to test positive, be hospitalized, be admitted to the intensive care unit, or die during the first year of the pandemic.[141][142]
Risk factors in these patients include poverty, low level of education, poor housing conditions, low family income, speaking in a language other than the national language, and household overcrowding.[135]
While the risk of diagnosis was higher in most ethnic minorities, once hospitalized, no clear inequalities in outcomes existed (except for the high risk of mortality in ethnic minorities in Brazil). This suggests that ethnic minority status is an important social determinant of COVID-related health outcomes, likely through association with other social determinants (e.g., housing, socioeconomic status, employment, general health status).[143] Racial disparities in outcomes may also be partially attributed to higher rates of comorbidities in certain ethnic groups.[144]
People in a long-term care facility are at increased risk for infection and severe disease.[126][145]
In the UK, care home residents represented approximately one third of the total number of deaths in England and Wales during the first wave of the pandemic; other countries reported a similar experience. This was likely due to shortages in personal protective equipment, a vulnerable population, and a lack of testing.[146] A study across four nursing homes found that 26% of residents died over a 2-month period, with all-cause mortality increasing by 203% compared with previous years. Approximately 40% of residents tested positive for SARS-CoV-2, and of these, 43% were asymptomatic and 18% had atypical symptoms.[147]
In the US, the 30-day all-cause mortality rate was 21% in a cohort study of more than 5000 nursing home residents. Older age, male sex, and impaired cognitive and physical function were independently associated with mortality.[148]
People with comorbidities are at increased risk for severe disease, and the more comorbidities, the greater the risk.[126][149]
In the UK, the most common comorbidities reported in a cohort study of more than 20,000 hospitalized patients were cardiac disease (31%), uncomplicated diabetes (21%), nonasthmatic chronic pulmonary disease (18%), and chronic kidney disease (16%).[150] Among 65,000 patients hospitalized in the UK, 68% reported at least one cardiometabolic condition on admission. Baseline cardiometabolic conditions were associated with an increased risk of in-hospital complications, and this risk increased in the presence of cardiometabolic multimorbidity.[151]
In the US, approximately 95% of hospitalized adults had at least one reported underlying medical condition, with the most common being hypertension, disorders of lipid metabolism, and obesity. Approximately 99% of patients who died had at least one underlying health condition. The strongest risk factors for death were obesity, anxiety and fear-related disorders, and diabetes, as well as the total number of underlying conditions.[152] It has been estimated that approximately 56% of adults, and 32% of young adults (ages 18-25 years), are at risk for severe disease because of the presence of at least one comorbidity.[153][154]
Globally, hypertension (21%), obesity (18%), and diabetes (18%) were the most prevalent comorbidities. Cancer, chronic kidney disease, diabetes, and hypertension were independently associated with mortality. Chronic kidney disease was statistically the most prominent comorbidity leading to death.[155] Metabolic syndrome is also significantly associated with a higher risk of mortality.[156]
Children with comorbidities including obesity, diabetes, chronic lung disease (not including asthma), heart disease, seizure disorders, and immunocompromised status had a high prevalence of severe disease.[157]
People with obesity (≥30 kg/m²) and people who are overweight (25-30 kg/m²) are at increased risk of infection and severe disease.[126] The risk of severe disease increases as body mass index (BMI) increases.[158] Obesity was highly prevalent in patients with COVID-19, with a pooled prevalence of 32% (37% in those requiring intensive care admission).[159]
Of the 2.5 million deaths reported globally by the end of February 2021, 2.2 million were in countries where more than half the population is classified as overweight. In countries where less than half the adult population is classified as overweight, the likelihood of death is around one tenth of the level seen in countries where more than half the population is classified as overweight.[160]
Evidence from meta-analyses found that patients who are obese have a significantly increased risk of infection, clinically severe disease, hospitalization, intensive care unit admission, need for mechanical ventilation, and mortality. Being overweight increases the risk of hospitalization, but not death.[161][162] However, the strength of association appears to have weakened over time.[162]
A cohort study in the UK found that the risk of severe outcomes (i.e., hospitalization, intensive care unit admission, death) increased progressively above a BMI ≥23 kg/m², independent of the excess risks of related diseases (e.g., diabetes). The relative risk was particularly notable in people age <40 years and those with black ethnicity. Every unit increase in BMI increased the risk of: hospital admission by 5% (above BMI ≥23 kg/m²); intensive care admission by 10% (any BMI); and death by 4% (BMI ≥28 kg/m²).[163]
A cohort study in the US found a nonlinear relationship between BMI and disease severity, with the lowest risk at BMIs near the threshold between healthy weight and overweight, then increasing with higher BMI.[164]
People with cardiovascular disease are at increased risk for severe disease.[126]
Preexisting cardiovascular disease is associated with adverse outcomes including disease severity, disease progression, and mortality in adults and children.[165][166]
Arrhythmias, coronary artery disease, and cardiovascular disease are significantly associated with intensive care unit admission. Heart failure, arrhythmias, coronary artery disease, and cardiovascular disease are also significantly associated with an increased risk of mortality.[167] Preexisting atrial fibrillation/atrial flutter was associated with a higher risk of intensive care admission, in-hospital mortality, and worse outcomes.[168][169] Coronary heart disease has also been associated with disease progression and severe/critical disease. The association is affected by the presence of hypertension; patients with coronary heart disease and hypertension had an increased risk of poor prognosis compared with those without hypertension.[170] Myocardial injury and peripheral artery disease are also associated with increased short-term mortality.[171][172]
People with risk factors for cardiovascular disease (e.g., hypertension, diabetes) are also at increased risk for severe disease and mortality (see below).[173][174]
People with type 1 or type 2 diabetes are at increased risk for severe disease.[126]
Diabetes is associated with a more than 2-fold increase in the risk for severe disease, and a slightly less than 2-fold increase in the risk for death. Diabetes is also associated with an increased risk for intensive care unit admission. Higher blood glucose levels (in the immediate and longer terms) are associated with worse outcomes. There is no evidence of difference in risk between people with new-onset and preexisting diabetes. Data are insufficient to determine whether diabetes predisposes people to infection. Data are mixed on whether type 1 diabetes predisposes a person to a higher risk from infection compared with type 2 diabetes. There are no data to suggest that diabetes increases the risk of severe disease in children and adolescents.[175][176] Variability across different regions in the world is significant and may skew overall trends.[177]
Risk factors for poor prognosis and higher mortality in patients with diabetes are similar to risk factors that exist in the general population and include older age, male sex, non-white ethnicity, socioeconomic deprivation, acute kidney injury, history of stroke or heart failure, and higher body mass index. Other more specific risk factors include prediabetes, poor glycemic control, higher glycosylated hemoglobin level, diabetic ketoacidosis, hyperglycemic hyperosmolar state, diabetic retinopathy, and insulin use.[178][179][180][181][182][183][184] Poor glycemic control in critically ill patients increases the risk of complications and mortality.[185] Studies that adjusted for age, sex, ethnicity, deprivation, and geographic location still found an increased risk for death in people with diabetes. There is little evidence regarding the role of comorbidities in increasing the risk of poor outcomes.[175]
Use of metformin, sodium–glucose cotransporter-2 (SGLT2) inhibitors, and glucagon-like peptide-1 (GLP-1) receptor agonists was associated with lower mortality in patients with type 2 diabetes. Dipeptidyl peptidase-4 (DPP-4) inhibitors and insulin have been associated with increased mortality. Sulfonylureas, thiazolidinediones, and alpha-glucosidase inhibitors did not appear to increase or decrease risk of mortality.[186][187] It is unclear whether these drugs have a protective effect, and further investigation is required.
Poor outcomes in these patients may be due to the syndromic nature of diabetes, the presence of comorbidities, impaired immune function, possible upregulation of enzymes that mediate viral invasion, and chronic inflammation coupled with the acute inflammatory reaction caused by SARS-CoV-2 resulting in a propensity for inflammatory storm.[188][189]
Infection is also associated with an elevated risk of new incident diabetes. The risk is associated with hospitalization and receiving intensive care.[190]
People with chronic lung diseases such as asthma, chronic obstructive pulmonary disease (COPD), interstitial lung disease, pulmonary embolism, pulmonary hypertension, tuberculosis, cystic fibrosis, and bronchiectasis are at increased risk for severe disease. People with bronchopulmonary dysplasia or alpha-1 antitrypsin deficiency may be at increased risk for severe disease; however, evidence is limited.[126] There is no clear evidence that people with asthma or COPD are at higher risk of infection.[191][192]
COPD: associated with an increased risk of hospitalization, intensive care unit admission, and mortality.[193][194] A national, multicenter prospective cohort study in the UK found that patients with COPD were less likely to receive critical care than patients without an underlying respiratory condition.[195]
Asthma: associated with similar (if not slightly improved) clinical outcomes compared with those without asthma. Pooled results from a large systematic review showed that, overall, asthma was not associated with severe outcomes (hospitalization, intensive care unit admission, mortality). However, evidence was of very low certainty, and results were limited by a lack of reporting on asthma severity, unexplained statistical heterogeneity, and imprecision. People with allergic asthma appear to be at a lower risk of severe outcomes, while people with asthma and concurrent COPD appear to be at a high risk of severe outcomes. Previous systematic reviews and meta-analyses have generated conflicting conclusions. Whether asthma is associated with an increased risk of infection or severe outcomes remains unclear.[196][197] Asthma was associated with an increased risk of hospitalization in children; however, use of inhaled corticosteroids was associated with a reduced risk of infection.[198]
Obstructive sleep apnea: associated with an increased risk for severe disease, intensive care admission, mechanical ventilation, and mortality, but not an increased risk of infection; however, evidence is limited.[199][200]
Cystic fibrosis: does not appear to be associated with an increased risk of infection; however, there is evidence that some patients may experience a more severe clinical course (e.g., post-transplantation).[201] Other risk factors for severe outcomes include FEV₁ <70%, age >40 years, diabetes, pancreatic insufficiency, underweight, and use of azithromycin.[202] Post-lung transplantation and cystic fibrosis-related diabetes were risk factors for severe disease in children.[198]
Active pulmonary tuberculosis: appears to be associated with an increased risk of severe disease and mortality.[203][204]
Interstitial lung disease: appears to be associated with an increased risk of severe disease and mortality.[205][206]
People with chronic kidney disease (including those receiving dialysis) are at increased risk for severe disease, and may be at higher risk for infection.[126][127]
Patients with chronic kidney disease had a significantly higher risk of hospitalization and all-cause mortality compared with people without chronic kidney disease. Patients with chronic kidney disease also had a higher risk of progressing to critical illness in the pooled analysis of included studies and subgroup analyses of studies with multivariable adjustment, although neither result achieved statistical significance.[207]
Incidence appears to be higher in patients receiving dialysis compared with those not requiring renal replacement therapy.[208] Patients with end-stage renal disease who were on renal replacement therapy also had an increased risk of intensive care unit admission, need for mechanical ventilation, and mortality.[209]
In the UK, data from a cross-sectional study found that the adjusted odds of a positive test were greater in patients with chronic kidney disease (32.9%) compared with those without (14.4%).[127]
Preexisting chronic kidney disease is an independent risk factor for developing acute kidney injury as a complication.[210]
People with chronic liver disease such as cirrhosis, metabolic dysfunction-associated fatty liver disease (nonalcoholic fatty liver disease), alcoholic liver disease, and autoimmune hepatitis are at increased risk for severe disease. People with hepatitis B or C may be at increased risk for severe disease; however, evidence is limited.[126]
Chronic liver disease has been associated with an increased risk for severe disease and mortality, but not an increased risk of infection.[211] Higher liver fibrosis scores are associated with worse prognosis.[212]
People with cirrhosis are at an increased risk of mortality. Cirrhotic patients had a 2.48-fold increased odds of mortality compared with noncirrhotic patients. Mortality risk is potentially higher in patients with more advanced cirrhosis.[213] Alcohol consumption is associated with an increased risk of severe disease, hospitalization, and intensive care unit admission.[214]
People with metabolic dysfunction-associated fatty liver disease are at increased risk for severe disease.[215] Disease severity has been associated with age <60 years and intermediate or high fibrosis-4 (FIB-4) scores.[216][217]
Pregnant women are at increased risk for severe disease.[126]
The overall prevalence in pregnant and recently pregnant women attending or admitted to hospital for any reason has been estimated to be 10%; however, the rate varies across studies and countries.[218][219] A meta-analysis of over 2500 pregnant women with confirmed disease found that 73.9% of women were in the third trimester; 50.8% were from black, Asian, or minority ethnic groups; 38.2% were obese; and 32.5% had chronic comorbidities.[220]
According to an analysis of approximately 400,000 women ages 15 to 44 years with symptomatic disease, pregnant women were more likely to be hospitalized, be admitted to the intensive care unit, receive invasive mechanical ventilation or extracorporeal membrane oxygenation, and die compared with nonpregnant women.[221]
Mortality rates vary significantly between geographic regions, with the highest rates in Sub-Saharan Africa and Latin America and the Caribbean, and by country income groups, with the highest rates in low-income countries.[222]
Risk factors for pregnant women developing severe disease, maternal morbidities, and adverse birth outcomes included age ≥30 years, mixed ethnicity, gestational diabetes, diabetes, hypertension, cardiovascular disease, kidney disease, overweight/obesity, HIV infection, pre-pregnancy underweight, and anemia.[223][224][225]
For more information on pregnancy-related complications, see Complications.
People who are current or former smokers are at increased risk for severe disease.[126]
Smoking is associated with severe or critical outcomes, and an increased risk of intensive care unit admission and mortality. The association appears to be more significant in former smokers compared with current smokers, and in younger people.[226] Current or former smokers (ever smokers) had a 16% higher risk of being hospitalized, a 44% higher risk of more severe disease, a 39% higher risk of mortality, and 45% higher risk for disease progression, compared with nonsmokers (never smokers).[227] This may be due to increased airway expression of the angiotensin-converting enzyme-2 receptor in smokers.[228] The risk of mortality in current smokers does not appear to vary by age; however, the risk drops significantly by age in former smokers.[229]
The World Health Organization (WHO) has reviewed the available evidence and concluded that smoking is associated with increased severity of disease and death in hospitalized patients.[230]
People with cancer (including hematologic malignancies) are at increased risk for infection and severe disease.[126][231]
Patients with cancer have an increased risk of infection, severe disease, intensive care unit admission, and mortality compared with the general population.[232] Hematologic malignancies were associated with the highest risk of severe disease and mortality (possibly explained by the greater degree of immunosuppression used in the treatment of these patients), followed by lung cancer. There is no clear association between treatment modality and mortality.[233]
The pooled in-hospital mortality risk in patients with cancer is 14.1%.[234] The pooled mortality in cancer patients admitted to the intensive care unit is 60.2%.[235] Mortality in cancer patients is affected by preexisting noncancer comorbidities, and is significantly higher in people with hypertension, cardiovascular disease, chronic obstructive pulmonary disease, and diabetes.[236]
Patients with recent cancer treatment (within 3 months before COVID-19 diagnosis) had a statistically significant increase in the risk of 30-day mortality, intensive care unit stay, and hospitalization compared with patients with COVID-19 without cancer. Patients with no recent cancer treatment had a similar risk of mortality and intensive care unit stay, and a lower risk of mechanical ventilation and hospitalization compared with patients without cancer.[237] Patients receiving immune checkpoint inhibitors do not appear to be at an increased risk of poor clinical outcomes compared with patients not receiving oncologic treatment or other cancer therapies, but evidence is limited.[238]
Children with cancer may be no more vulnerable to infection compared with children without cancer. Limited data show that the overall morbidity in pediatric patients with cancer is low, with only 5% requiring hospitalization for symptoms.[239] In the largest international cohort study to date, 20% of children with cancer developed severe or critical disease, but most patients recovered without advanced support. Approximately 35% of children were asymptomatic. Lymphopenia and neutropenia were associated with more severe disease.[240] Overall survival in children with cancer is very high (99.4%), and there was no significant difference in the risk of hospitalization or intensive care unit admission between hematologic malignancies and solid tumors in children.[241] Limited evidence suggests that no severe complications were associated with continuation of chemotherapy in children who test positive for SARS-CoV-2.[242]
People with cerebrovascular disease are at increased risk for severe disease.[126]
Patients with a history of cerebrovascular disease were more likely to progress to adverse outcomes compared with patients without a history of cerebrovascular disease.[243] Patients with preexisting cerebrovascular disease had 2.67-fold higher odds of poor outcomes including intensive care admission, mechanical ventilation, and mortality.[244] Previous stroke was significantly associated with severe disease, intensive care unit admission, mechanical ventilation, and mortality.[245]
People with mental health disorders such as mood disorders (e.g., depression) and schizophrenia-spectrum disorders are at increased risk for severe disease.[126]
People with preexisting mental health disorders have an increased risk of hospitalization and mortality compared with patients without mental health disorders.[246][247]
People with preexisting schizophrenia may be at increased risk of mortality. Risk factors include older age and a history of smoking.[248]
People with an immunocompromised state from solid organ or blood stem cell transplant are at increased risk for severe disease.[126]
Solid organ transplant recipients are at increased risk for hospitalization, intensive care unit admission, and mortality. However, the increased rate of hospitalization may reflect a preferred management strategy of closer inpatient monitoring in these patients rather than being an indicator of disease severity. No increased risk in mortality was found compared with the general population when adjusted for demographic and clinical features and disease severity.[249]
Hematopoietic stem cell transplant (HSCT) recipients are at increased risk for mortality. The mortality rate was slightly higher in allo-HSCT recipients compared with auto-HSCT recipients, but this difference was not statistically significant. Risk factors for higher mortality included older age, immunosuppressive therapy, graft-versus-host disease, and elevated inflammatory markers with lymphopenia.[250]
People with disabilities including Down syndrome, cerebral palsy, congenital malformations, learning disabilities, attention deficit/hyperactivity disorder, intellectual and developmental disabilities, and spinal cord injuries (among others) are at increased risk for severe disease.[126]
In the UK, a cohort study found a 4-fold increased risk for hospitalization and a 10-fold increased risk for mortality in people with Down syndrome.[251] This may possibly be due to the presence of immune dysfunction, congenital heart disease, and pulmonary pathology. Also, people with Down syndrome often have associated obesity and obstructive sleep apnea, both of which are risk factors for worse outcomes. The mortality rate was higher in those over age 40 years in one systematic review.[252]
Another study in the UK found that adults with learning disability and those with Down syndrome or cerebral palsy have markedly increased risks of hospital admission and death over and above the risks observed for non-COVID-19 causes of death.[253]
The risk of death was higher for disabled people (including learning disability, neurologic conditions, and frailty) compared with nondisabled people during the first two waves of the pandemic. Relative risks were high among younger disabled people, disabled women, and people with greater levels of activity limitation. Adverse socioeconomic, demographic, and health-related risk factors accounted for some of the elevated risk.[254]
People with dementia may be at increased risk for infection and are at increased risk for severe disease.[126][255]
Older adults with dementia are at a higher risk of mortality in the short term. Dementia patients are more likely to be vulnerable to having diseases such as hypertension, diabetes, and pneumonia, and be immunocompromised. The pooled mortality rate of patients with dementia was 39% compared with 20% in older adults without dementia.[256]
In the UK, over one quarter of people who died with COVID-19 from March to June 2020 had dementia. Dementia and Alzheimer disease was the most common main preexisting health condition in deaths involving COVID-19 between March and June 2020.[257]
A retrospective case-control study of electronic patient health records in the US found that patients with dementia were at increased risk of infection compared with patients without dementia. They also had significantly worse outcomes (6‐month hospitalization risk and mortality risk) compared with patients with dementia but no COVID‐19 and patients with COVID‐19 but no dementia. The highest risk was seen in patients with vascular dementia.[258]
People with Parkinson disease may be at increased risk for infection and are at increased risk for severe disease.[126][259][260][261]
Risk factors for infection may include obesity, pulmonary disease, and hospitalization. Vitamin D supplementation was associated with a lower risk of infection.[260]
Parkinson disease was associated with severe disease, poor in-hospital outcomes, and mortality in one meta-analysis. However, the evidence for an association is still unclear. The association was influenced by age, but not by sex or the presence of dementia, hypertension, or diabetes.[259]
Patients may experience substantial worsening of parkinsonian symptoms.[262]
People who are immunocompromised are at increased risk for severe disease.[126] People with immunocompromising conditions have a higher risk of hospitalization and mortality.[263]
This includes people with a history of primary immune deficiencies or prolonged use of corticosteroids or other immunosuppressant medications.
Once hospitalized, immunocompromised people are at increased risk for intensive care unit admission and death, irrespective of vaccination status, compared with nonimmunocompromised patients (after adjusting for differences in clinical and demographic characteristics).[264]
Current data do not strongly suggest that medications associated with the treatment of immune-mediated inflammatory diseases increase the risk of infection or severe disease, with the exception of corticosteroids and rituximab.[265] Glucocorticoid exposure of ≥10 mg/day (prednisone) has been associated with a higher odds of hospitalization in patients with rheumatologic disease.[266] Patients treated with cyclosporine/tacrolimus also had an increased risk for hospitalization; however, it was not clear whether the increased risk is related to the drug itself, the underlying condition for which the patient is treated, or other factors.[267]
Immunosuppressed patients are not at significantly increased risk of infection compared with the general population.[268]
Also see HIV infection and Autoimmune disease below.
People living with HIV are at increased risk for severe disease.[126]
Retrospective studies have found that while people with HIV do not appear to be at increased risk of infection, they are at increased risk for poor outcomes (i.e., severe disease, hospitalization, mortality) compared with people living without HIV infection. The risk of severe disease and hospitalization increased with progression of HIV disease stage.[269][270][271][272] However, there is some evidence that suggests that HIV patients at advanced stages (stage 3 or 4) may manifest less severe symptoms and have reduced mortality. This may be due to the inability of HIV-positive individuals' immune systems to provoke the cytokine storm that usually causes poor clinical outcomes in COVID-19 patients.[273]
Evidence from individual studies, systematic reviews, and meta-analyses is conflicting. Some have found that HIV infection was not associated with composite poor outcome or mortality, while others have found that people living with HIV infection have an increased risk for infection and mortality compared with people without HIV.[274]
The World Health Organization (WHO) states that HIV infection appears to be a significant independent risk factor for severe or critical disease at hospital admission and in-hospital mortality. HIV infection was independently associated with a higher risk of mortality compared with the HIV-negative population after adjusting for age, sex, disease severity, and underlying conditions. Age >65 years, male sex, and the presence of diabetes or hypertension were risk factors for severe or critical illness at hospital admission, as well as in-hospital mortality. Data were predominantly from South Africa, which may limit the generalizability of the results.[275][276] Other risk factors for severe illness include coexisting cardiovascular disease, respiratory disease, and chronic kidney disease.[277]
People who are not physically active are at increased risk for severe disease.[126]
Data indicate an association between physical inactivity and an increased risk of hospitalization and mortality, and possibly an association between physical inactivity and increased risk of ventilation. There are limited data on the association between physical inactivity and intensive care unit admission and intubation.[278][279][280] Moderate-to-vigorous physical activity reduced the odds of infection, hospitalization, and mortality compared with no physical activity.[281]
People with sickle cell disease or thalassemia may be at increased risk for severe disease; however, evidence is limited.[126] People with sickle cell disease or trait are at increased risk of hospitalization and mortality.[282]
Patients with hemoglobinopathy had an increased risk of severe disease and mortality compared with the general population. Mortality among patients with hemoglobinopathy was 6.9%. Respiratory and cardiovascular comorbidities were significant predictors of mortality.[283][284]
In the UK, patients with sickle cell disease were found to have a 4-fold increased risk for hospitalization and a 2.6-fold increased risk for death. Sickle cell trait was also associated with increased risks for both outcomes, albeit to a lesser extent.[285]
In the US, among 178 patients with sickle cell disease (mean patient age <40 years), 69% were hospitalized, 11% were admitted to intensive care, and 7% died.[286] Infection can cause acute chest syndrome in patients with sickle cell disease.[287][288]
People with hemophilia may be at increased risk for severe disease; however, evidence is limited.[126]
People with hemophilia were more likely to have moderate disease and be admitted to hospital compared with those without hemophilia in one retrospective study. Hemophilia increased the risk of bleeding in patients with COVID-19, and was not protective against venous thromboembolism despite the hypocoagulable state. There was no significant difference between mortality rates, or other markers of disease severity (e.g., intensive care unit admission, need for oxygen).[289]
People with hypertension may be at increased risk for severe disease; however, evidence is limited.[126]
Almost all available evidence suggests that hypertension increases the risk of severe disease or mortality, although it was sometimes unclear whether this was independent of other risk factors. There were no systematic reviews or meta-analyses studying whether people with hypertension were at greater risk of infection.[290][291]
Hypertension has been associated with increased poor composite outcome, including mortality, severe disease, acute respiratory distress syndrome, need for intensive care admission, and disease progression.[292][293] Patients with hypertension have a 2.98-fold higher risk of severe disease, a 1.82-fold higher risk of critical disease, and a 2.17 to 2.88-fold higher risk of fatality compared with patients without hypertension.[294][295]
Initially, there was a concern that people on ACE inhibitors or angiotensin-II receptor antagonists may be at increased risk for infection or severe disease due to upregulation of ACE2 receptor expression.[296] However, high-certainty evidence suggests that use of these drugs is not associated with severe disease, and there is no association between the use of these medications and a positive SARS-CoV-2 test result among symptomatic patients.[297][298]
People with epilepsy may be at increased risk for severe disease; however, evidence is limited.[126]
Older adults (age ≥65 years) with epilepsy had a significantly higher risk of inhospital mortality and longer length of hospital stay compared with those without epilepsy in one retrospective study.[299]
People with substance use disorders may be at increased risk for severe disease; however, evidence is limited.[126] This includes alcohol, opioid, or cocaine use disorder.
People with substance abuse disorders, especially those using drugs that affect the respiratory and cardiovascular systems, may be vulnerable to the adverse respiratory effects of COVID-19. Cohort studies have found substance use disorders were associated with increased hospitalization, intensive care unit admission, ventilator use, and mortality.[300][301] A systematic review and meta-analysis found that people with opioid use disorder have an increased risk of hospitalization, intensive care unit admission, and mortality.[302]
Children with certain underlying conditions may be at increased risk for severe disease; however, evidence is limited.[126] The presence of at least one comorbidity was associated with a markedly increased risk for critical disease in children.[303]
These conditions include obesity, diabetes, asthma and chronic lung disease, immunosuppression, and sickle cell disease. Children may also be at risk if they are medically complex; have serious genetic, neurologic, or metabolic disorders; or have congenital heart disease.[126]
A cross-sectional study of over 43,000 children in the US found that the most commonly documented underlying conditions were obesity, asthma, neurodevelopmental disorders, anxiety and fear-related disorders, and depressive disorders. Children with type 1 diabetes, cardiac and circulatory congenital anomalies, obesity, hypertension, epilepsy, neuropsychiatric disorders, and asthma as well as children with chronic disease had higher risk of hospitalization and severe disease. Limited data suggest that children with congenital heart disease might be at increased risk of severe disease.[304]
People with vitamin D deficiency may be at higher risk for infection and severe disease; however, evidence is limited.
Meta-analyses have found that low serum vitamin D level is significantly associated with a higher risk of infection, and increased risk for severe disease, hospitalization, and mortality in both adults and children.[305][306][307][308][309][310][311] However, it is unclear whether these associations were statistically significant and the certainty of evidence is very low.[312][313] When the analyses included studies with adjustments for confounders, vitamin D deficiency was not associated with an increased risk of mortality.[314]
A meta-analysis and GRADE assessment of cohort studies and randomized controlled trials found that current evidence suggests that vitamin D deficiency is not significantly linked to susceptibility to infection or death, and vitamin D supplementation did not significantly improve clinical outcomes. However, the overall quality of evidence was low.[315]
People taking proton-pump inhibitors (PPIs) may be at increased risk for infection and severe disease; however, evidence is limited.[316]
Data on whether PPI use increases the risk for infection is conflicting. The largest meta-analysis to date found that PPI use was marginally associated with a nominal, but statistically significant, increase in the risk of infection, as well as an increased risk of severe infection and mortality.[316]
Current or regular users of PPIs were more likely to have severe outcomes compared with non-PPI users. Also, current PPI users were more likely to be hospitalized for longer compared with non-PPI users, although this was not statistically significant. Past use of PPIs is not associated with increased susceptibility to infection or severe outcomes.[317]
While corticosteroids are used for the treatment of COVID-19 to reduce mortality, long-term use of oral corticosteroids for another condition prior to the development of COVID-19 was found to increase hospitalization, intensive care unit admission, and mortality compared with nonusers, according to one population-based cohort study.[318]
People with autoimmune disease (including rheumatic and musculoskeletal diseases) may be at higher risk for infection and severe disease; however, evidence is limited.[319][320]
Current data do not strongly suggest that the presence of an immune-mediated inflammatory disease increases the risk of infection or severe disease. The increased risk reported in some studies may be due to comorbidities associated with immune-mediated inflammatory diseases or medications the patient is taking (corticosteroids, rituximab). Increased rates of hospitalization in these patients were not associated with increased rates of death.[265] Tumor necrosis factor (TNF)-alpha inhibitor monotherapy was associated with a lower risk of hospitalization or death among patients with immune-mediated inflammatory disorders compared with other treatment regimens (e.g., methotrexate, azathioprine, Janus kinase [JAK] inhibitors).[321] There was no increased risk of mechanical ventilation or in-hospital mortality for other rheumatologic, antineoplastic, or antimetabolite therapies examined in one cohort study, with the exception of rituximab.[322]
People with inflammatory arthritis may be at higher risk for infection and severe disease; however, evidence is limited.
Evidence does not show a strong association between inflammatory arthritis (e.g., rheumatoid arthritis, spondyloarthritis) and risk of infection or adverse outcomes such as hospitalization, intensive care unit admission, need for mechanical ventilation, or death. However, evidence is conflicting. Some studies do report an increased risk of adverse outcomes, but the studies had limitations.[265]
People with inflammatory bowel disease may be at higher risk for infection and severe disease; however, evidence is limited.
Prevalence in patients with inflammatory bowel disease appears to be low.[323][324] Inflammatory bowel disease was not associated with an increased risk of hospitalization, intensive care unit admission, or mortality.[325] Evidence suggests that the risk profile for infection and severe disease is similar to the general population if patients have good disease control and do not use corticosteroids.[265] Corticosteroid use was associated with an increased risk for severe disease and intensive care unit admission, but not mortality.[326] Higher disease activity and flares may lead to increased susceptibility to infection and worse outcomes.[327]
Patient outcomes (hospitalization, intensive care unit admission, and mortality) were worse in ulcerative colitis (compared with Crohn disease), and patients on corticosteroids, thiopurines, aminosalicylates, or combination therapy. Outcomes were better in patients on biologic agents.[323][325][328][329][330]
A risk calculator has been developed that predicts which patients with inflammatory bowel disease are at higher risk of adverse outcomes.[331]
People with connective tissue disease may be at higher risk for infection and severe disease; however, evidence is limited.
Several studies suggest an increased risk of infection in patients with connective tissue disorders (e.g., systemic lupus erythematosus, Sjogren syndrome, systemic sclerosis, polymyositis and dermatomyositis) compared with the general population and patients with other immune-mediated inflammatory diseases. This is possibly due to the widespread use of corticosteroids in these patients. There is a lack of data regarding outcomes and evidence is conflicting.[265]
Patients with lupus nephritis were at increased risk of developing severe or critical disease.[332]
People with vasculitis may be at higher risk for infection and severe disease; however, evidence is limited.
Corticosteroid use, older age, male sex, moderate or severe disease activity, comorbidities (e.g., respiratory disease), and rituximab or cyclophosphamide use were associated with severe outcomes, based on limited data.[333][334]
People with multiple sclerosis may be at higher risk for infection and severe disease; however, evidence is limited.
Neurologic disability, older age, black race, cardiovascular comorbidities, recent treatment with corticosteroids, and obesity were risk factors for severe disease and mortality.[335][336] The risk of severe disease was higher in patients with progressive disease compared with those with relapsing-remitting disease. Use of anti-CD20 therapies was associated with an increased risk of severe disease in patients with relapsing-remitting disease.[337]
Current evidence does not suggest that multiple sclerosis significantly increases the mortality rate. Highest hospitalization and mortality rates were in patients who were not on disease-modifying therapies, followed by those who were on B cell-depleting therapies (e.g., rituximab, ocrelizumab).[338]
Current evidence does not suggest that patients with multiple sclerosis who are infected with SARS-CoV-2 are at an increased risk of relapse.[339]
People with hypothyroidism may be at higher risk of severe disease; however, evidence is limited.[340][341]
Thyroid disorders (hypothyroidism and unspecified thyroid abnormalities, but not hyperthyroidism) are associated with a higher risk of poor outcomes including severe disease, hospitalization, intensive care unit admission, and mortality. This association was significantly associated with increasing age and presence of hypertension.[342][343]
Gout appears to be associated with an increased risk for infection and mortality; however, evidence is limited.[344]
A UK Biobank population-based study of over 15,000 people with gout found that gout was associated with an increased risk for diagnosis of COVID-19 and COVID-19-related death, independent of the metabolic comorbidities of gout. Women were at a higher risk of death compared with men. There were no significant differences in the risk of COVID-19-related death according to prescription of colchicine or urate-lowering therapy.[344] Evidence is limited and further research is required.
Dyslipidemia appears to be associated with an increased risk for severe disease and mortality; however, evidence is limited.[345][346][347]
The association was stronger in males, older age, and those with hypertension.[348]
Initially there was a concern that people on statins may be at increased risk of infection or more severe disease, as statins have been shown to increase ACE2 expression in animals and may promote the activation of the inflammatory pathway in acute respiratory distress syndrome.[296] However, so far, studies do not support this hypothesis, and studies have shown a protective effect (lower risk of mortality or severe disease).[349] Findings from the American Heart Association’s COVID-19 Cardiovascular Disease Registry report that patients taking statins prior to hospitalization had substantially lower odds of death, primarily among individuals with a history of cardiovascular disease and/or hypertension.[350] Similar findings have been reported from a Swedish registry study.[351]
Surgical mortality and complications may be higher in patients with COVID-19 compared with patients without COVID-19.[352]
A retrospective study of 34 patients in China who underwent elective surgeries during the incubation period of COVID-19 found that all patients developed pneumonia after surgery. Approximately 44% of these patients required admission to the intensive care unit, and 20% died.[353]
Postoperative pulmonary complications occur in half of patients with perioperative SARS-CoV-2 infection, and are associated with higher mortality, particularly in men and those ages 70 years and over.[354]
People with blood group A may be at increased risk for infection and mortality, and people with blood groups B and AB may be at increased risk for infection; however, evidence is limited.[355][356][357][358]
Blood group O appears to be protective against infection; however, evidence is of low/very low quality. People who are Rh-positive were more vulnerable to infection compared with those who were Rh-negative.[355][359][360]
A genome-wide association study found that patients with blood group A are at 45% increased risk of respiratory failure compared with other blood groups. It also found a protective effect in blood group O. Two chromosomal loci were associated with respiratory failure, and one of these coincided with the ABO blood group locus.[361] The SARS-CoV-2 receptor-binding domain directly binds the blood group A antigen expressed on respiratory epithelial cells, directly linking blood group A and SARS-CoV-2.[362]
There is limited evidence that gut and lung microbiota dysfunction may be implicated in the pathogenesis of COVID-19.[363]
A dysbiotic gut bacterial profile has been noted during the acute and recovery phases.[364] Patients appear to have a depletion of beneficial commensals (e.g., Eubacterium ventriosum, Eubacterium rectale, Faecalibacterium prausnitzii, Roseburia and Lachnospiraceae taxa) and an overgrowth of opportunistic pathogens (e.g., Clostridium hathewayi, Actinomyces viscosus, Bacteroides nordii) during hospitalization.[365][366][367] Associations between gut microbiota composition, levels of cytokines, and inflammatory markers in patients with COVID-19 suggest that the gut microbiome is involved in disease severity, possibly via modulating host immune responses. Gut dysbiosis after disease resolution may contribute to persistent symptoms.[368]
People with zinc deficiency may have a higher risk of mortality; however, evidence is limited and the exact role of zinc in COVID-19 is not well understood.
One meta-analysis found that patients with zinc deficiency had a higher risk of mortality and symptomatology compared with those without zinc deficiency. No association was found between zinc levels and the severity of symptoms. However, further research is required.[369]
Use of this content is subject to our disclaimer
