Resumen
Definición
Anamnesis y examen
Principales factores de diagnóstico
- cough
- shortness of breath
- sputum production
- exposure to risk factors
Otros factores de diagnóstico
- barrel chest
- hyperresonance on percussion
- distant breath sounds on auscultation
- poor air movement on auscultation
- wheezing on auscultation
- coarse crackles
- tachypnea
- asterixis
- distended neck veins
- lower-extremity swelling
- fatigue
- weight loss
- muscle loss
- headache
- pursed lip breathing
- cyanosis
- loud P2
- hepatojugular reflux
- hepatosplenomegaly
- clubbing
Factores de riesgo
- cigarette smoking
- advanced age
- genetic factors
- lung growth and development
- white ancestry
- exposure to air pollution
- exposure to burning solid or biomass fuel
- occupational exposure to dusts, chemicals, pesticides, vapors, fumes, or gases
- male sex
- low socioeconomic status
- rheumatoid arthritis
Pruebas diagnósticas
Primeras pruebas diagnósticas para solicitar
- spirometry
- standardized symptoms score
- pulse oximetry
- ABG
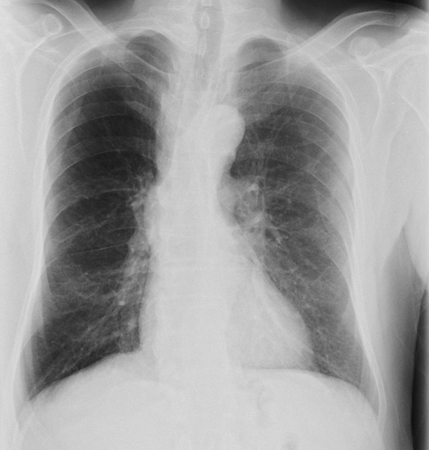
- Chest x-ray
- CBC
Pruebas diagnósticas que deben considerarse
- pulmonary function tests
- chest CT scan
- serial peak flow measurement
- sputum culture
- alpha-1 antitrypsin level
- exercise testing
- sleep study
- respiratory muscle function
- ECG
- echocardiogram
- blood eosinophil count
Algoritmo de tratamiento
Colaboradores
Autores
Manoochehr Abadian Sharifabad, MD

Fountain Valley Regional Medical Center
Fountain Valley
CA
Divulgaciones
MAS declares that he has no competing interests.
Agradecimientos
Dr Manoochehr Abadian Sharifabad would like to gratefully acknowledge Dr Jonathan P. Parsons and Dr Michael Ezzie, the previous contributors to this topic.
Divulgaciones
JPP has contributed at speakers' bureaus for GlaxoSmithKline, Inc., Schering-Plough, Inc., and AstraZeneca, Inc. ME declares that he has no competing interests.
Revisores por pares
Hormoz Ashtyani, MD, FCCP
Hackensack University Medical Center
Hackensack
NJ
Divulgaciones
HA declares that he has no competing interests.
Alejandro Comellas, MD
Clinical Professor of Internal Medicine-Pulmonary, Critical Care and Occupational Medicine
University of Iowa
Iowa City
IA
Divulgaciones
AC has received payments as a consultant for GSK and AstraZeneca. He also serves as a nonpaid consultant for VIDA.
William Janssen, MD
Assistant Professor of Medicine
National Jewish Medical and Research Center
University of Colorado Health Sciences Center
Denver
CO
Divulgaciones
WJ declares that he has no competing interests.
Francis Thien, MD, FRACP, FCCP
Associate Professor
Director of Respiratory Medicine
Eastern Health & Monash University
Victoria
Australia
Divulgaciones
FT declares that he has no competing interests.
Agradecimiento de los revisores por pares
Los temas de BMJ Best Practice se actualizan de forma continua de acuerdo con los desarrollos en la evidencia y en las guías. Los revisores por pares listados aquí han revisado el contenido al menos una vez durante la historia del tema.
Divulgaciones
Las afiliaciones y divulgaciones de los revisores por pares se refieren al momento de la revisión.
Referencias
Artículos principales
Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2025 report. 2025 [internet publication].Texto completo
National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management. Jul 2019 [internet publication].Texto completo
Nici L, Mammen MJ, Charbek E, et al. Pharmacologic management of chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020 May 1;201(9):e56-69.Texto completo Resumen
Jacobs SS, Krishnan JA, Lederer DJ, et al. Home oxygen therapy for adults with chronic lung disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020 Nov 15;202(10):e121-41. [Erratum in: Am J Respir Crit Care Med. 2021 Apr 15;203(8):1045-6.]Texto completo Resumen
Artículos de referencia
Una lista completa de las fuentes a las que se hace referencia en este tema está disponible aquí.
El uso de este contenido está sujeto a nuestra cláusula de exención de responsabilidad