The goals of treatment of COPD are to prevent and control symptoms, to reduce the severity and number of exacerbations, to improve respiratory capacity for increased exercise tolerance, and to reduce mortality.[1]Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2025 report. 2025 [internet publication].
https://goldcopd.org/2025-gold-report
Systematic reviews report a modest reduction in rate of decline of FEV₁ among patients receiving pharmacologic therapy.[84]Celli BR, Anderson JA, Cowans NJ, et al. Pharmacotherapy and lung function decline in patients with chronic obstructive pulmonary disease. A systematic review. Am J Respir Crit Care Med. 2021 Mar 15;203(6):689-98.
https://www.atsjournals.org/doi/10.1164/rccm.202005-1854OC
http://www.ncbi.nlm.nih.gov/pubmed/32966751?tool=bestpractice.com
[85]Yang IA, Ferry OR, Clarke MS, et al. Inhaled corticosteroids versus placebo for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2023 Mar 27;(3):CD002991.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD002991.pub4/full
http://www.ncbi.nlm.nih.gov/pubmed/36971693?tool=bestpractice.com
Further research is needed to find out which patients are most likely to benefit.
There is a stepwise approach to therapy, and treatment should be individualized for general health status and comorbid conditions. If a patient with COPD has concomitant asthma, they should primarily be managed according to asthma guidelines.[1]Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2025 report. 2025 [internet publication].
https://goldcopd.org/2025-gold-report
See Asthma in adults. For details on the management of alpha-1 antitrypsin deficiency, see Alpha-1 antitrypsin deficiency.
The therapeutic approach involves reducing risk factor exposure, appropriate assessment of disease, patient education, pharmacologic and nonpharmacologic management of stable COPD, and prevention and treatment of acute COPD exacerbations.
The World Health Organization (WHO) has specified a minimum set of interventions for the management of stable COPD in primary care.
WHO: package of essential noncommunicable (PEN) disease interventions for primary health care
Opens in new window
Continuous assessment and monitoring of disease
Ongoing monitoring and assessment in COPD ensures that the goals of treatment are being met. Quality of life and patients' sense of wellbeing will improve, and hospital admissions will be significantly decreased, when self- or professional monitoring of disease is being utilized.[86]Lemmens KM, Nieboer AP, Huijsman R. A systematic review of integrated use of disease-management interventions in asthma and COPD. Respir Med. 2009 May;103(5):670-91.
http://www.ncbi.nlm.nih.gov/pubmed/19155168?tool=bestpractice.com
Such assessment of the medical history should include:
Exposure to risk factors and preventive measures:
Tobacco smoke
Indoor and outdoor air pollution
Occupational exposures (fumes, dust, etc.)
Influenza and pneumococcal vaccination
Disease progression and development of complications:
Pharmacotherapy and other medical treatment:
How often rescue inhaler is used
Any new medicines
Compliance with medical regimen
Ability to use inhalers properly
Adverse effects
Exacerbation history:
Urgent care or emergency room visits
Recent oral corticosteroid bursts
Frequency, severity, and likely causes of exacerbations should be evaluated
Comorbidities:
In addition, objective assessment of lung function should be obtained yearly, or more frequently if there is a substantial increase in symptoms.
One Cochrane review found that integrated disease management (IDM), in which several healthcare providers (physical therapist, pulmonologist, nurse, etc.) work together with patients, probably results in improvement in disease‐specific quality of life, exercise capacity, hospital admissions, and hospital days per person.[87]Poot CC, Meijer E, Kruis AL, et al. Integrated disease management interventions for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2021 Sep 8;(9):CD009437.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD009437.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/34495549?tool=bestpractice.com
[  ]
What are the effects of integrated disease management (IDM) interventions for people with chronic obstructive pulmonary disease (COPD)?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.3877/fullShow me the answer[Evidence A]e42ce1ee-fdf1-4c06-93f6-e00ea3a5b9c6ccaAWhat are the effects of integrated disease management (IDM) interventions for people with chronic obstructive pulmonary disease (COPD)?
]
What are the effects of integrated disease management (IDM) interventions for people with chronic obstructive pulmonary disease (COPD)?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.3877/fullShow me the answer[Evidence A]e42ce1ee-fdf1-4c06-93f6-e00ea3a5b9c6ccaAWhat are the effects of integrated disease management (IDM) interventions for people with chronic obstructive pulmonary disease (COPD)?
Chronic management: stepwise therapy according to GOLD group
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommend that initial treatment is determined by the patient’s GOLD group at diagnosis:[1]Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2025 report. 2025 [internet publication].
https://goldcopd.org/2025-gold-report
Rescue short-acting bronchodilators should be prescribed to all patients for immediate symptom relief. Failure to respond to short-acting bronchodilator may signify an acute exacerbation.
For group A patients (few symptoms [Modified British Medical Research Council {mMRC} 0-1 or COPD Assessment Test {CAT} <10] and low risk of exacerbations [0-1 exacerbations per year, not requiring hospitalization]), a short-acting or a long-acting bronchodilator is offered first-line. Long-acting beta-2 agonists (LABAs) and long-acting muscarinic antagonists (LAMAs) are preferred over short-acting bronchodilators, except for patients with only very occasional dyspnea.
For group B patients (more symptoms [mMRC ≥2 or CAT ≥10] and low risk of exacerbations [0-1 exacerbations per year, not requiring hospitalization]), LABA/LAMA combination treatment should be offered first-line in the absence of issues with adverse effects or availability. Otherwise, monotherapy with either a LAMA or a LABA may be prescribed. There is no evidence to recommend one class of long-acting bronchodilator over another for bronchodilator monotherapy. The choice should depend on the patient's perception of symptom relief. Patients in group B may have comorbidities that add to their symptoms and impact their prognosis, and so any potential comorbidities should be considered and investigated.
For group E patients (high risk of exacerbations [≥2 exacerbations per year, or ≥1 requiring hospitalization] and any level of symptoms), LABA/LAMA combination treatment is first-line therapy in the absence of issues with adverse effects or availability. Addition of an inhaled corticosteroid (ICS) to a LABA/LAMA combination may be considered if the patient's blood eosinophil count is ≥300 cells/microliter (triple therapy). Use of ICS with LABA alone is not recommended.
[Figure caption and citation for the preceding image starts]: Initial pharmacologic management of COPDGlobal Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2025 report); reproduced with permission [Citation ends].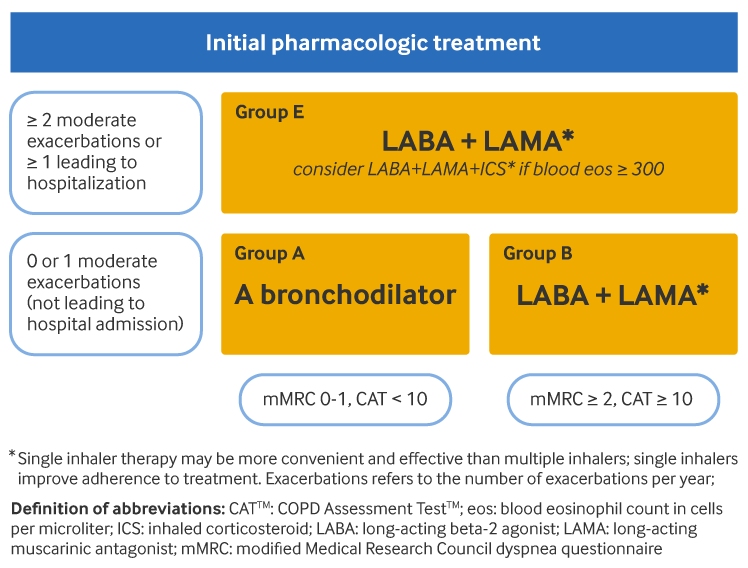
Further treatment is determined by the patient's dyspnea/exercise limitation symptom burden and frequency of exacerbations after review and is independent of the patient's GOLD group at diagnosis. GOLD recommends different treatment pathways depending on whether the primary treatment goal is relieving dyspnea/exercise limitation symptoms or reducing exacerbations. If treatment is required for both purposes, clinicians should follow the exacerbation pathway.[1]Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2025 report. 2025 [internet publication].
https://goldcopd.org/2025-gold-report
Before any adjustment in treatment, patients should be reviewed for symptoms and exacerbation risk, and their inhaler technique and treatment adherence assessed. The role of nonpharmacologic treatment should also be assessed. If the patient's response to initial treatment is appropriate, then the initial treatment can be maintained. Adjustment of pharmacologic treatment can consist of escalation or de-escalation of therapy, as well as switching inhaler devices or molecules within the same drug class. If treatment is changed, then clinicians should review the patient for a clinical response, and for any potential adverse effects.[1]Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2025 report. 2025 [internet publication].
https://goldcopd.org/2025-gold-report
Recommended escalation therapy for patients with persistent dyspnea/exercise limitation after initial therapy is as follows:[1]Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2025 report. 2025 [internet publication].
https://goldcopd.org/2025-gold-report
Patients taking long-acting bronchodilator monotherapy should start a second long-acting bronchodilator
If symptoms persist following addition of a second long-acting bronchodilator
consider switching the inhaler device or molecules
implement or escalate nonpharmacologic treatment (e.g., pulmonary rehabilitation)
consider adding ensifentrine (a phosphodiesterase-3 [PDE-3] and PDE-4 inhibitor)
Dyspnea due to other causes should be considered, investigated, and treated. Inhaler technique and adherence should also be reassessed, as these may have led to an inadequate response to treatment.
Recommended escalation therapy for patients with persistent exacerbations after initial therapy is as follows:[1]Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2025 report. 2025 [internet publication].
https://goldcopd.org/2025-gold-report
Patients taking long-acting bronchodilator monotherapy should increase therapy to LABA/LAMA. Blood eosinophil counts can identify patients who are more likely to respond to ICS.[76]Bafadhel M, Peterson S, De Blas MA, et al. Predictors of exacerbation risk and response to budesonide in patients with chronic obstructive pulmonary disease: a post-hoc analysis of three randomised trials. Lancet Respir Med. 2018 Feb;6(2):117-26.
http://www.ncbi.nlm.nih.gov/pubmed/29331313?tool=bestpractice.com
[77]Harries TH, Rowland V, Corrigan CJ, et al. Blood eosinophil count, a marker of inhaled corticosteroid effectiveness in preventing COPD exacerbations in post-hoc RCT and observational studies: systematic review and meta-analysis. Respir Res. 2020 Jan 3;21(1):3.
https://respiratory-research.biomedcentral.com/articles/10.1186/s12931-019-1268-7
http://www.ncbi.nlm.nih.gov/pubmed/31900184?tool=bestpractice.com
[78]Oshagbemi OA, Odiba JO, Daniel A, et al. Absolute blood eosinophil counts to guide inhaled corticosteroids therapy among patients with COPD: systematic review and meta-analysis. Curr Drug Targets. 2019;20(16):1670-9.
http://www.ncbi.nlm.nih.gov/pubmed/31393244?tool=bestpractice.com
Escalation to triple therapy with LABA/LAMA/ICS may be considered for patients on long-acting bronchodilator monotherapy if their peripheral eosinophil count is ≥300 cells/microliter. ICS is unlikely to be beneficial in patients whose blood eosinophil count is <100 cells/microliter.
Patients who take LABA/LAMA and whose blood eosinophils are ≥100 cells/microliter should escalate to triple therapy with LABA/LAMA/ICS. Multiple studies support triple therapy with LABA/LAMA/ICS as being superior to single- or double-agent therapy with LABA/LAMA or LABA/ICS regarding rate of moderate to severe COPD exacerbations and rate of hospitalization.[80]Cazzola M, Rogliani P, Calzetta L, et al. Triple therapy versus single and dual long-acting bronchodilator therapy in COPD: a systematic review and meta-analysis. Eur Respir J. 2018 Dec 13;52(6):1801586.
https://erj.ersjournals.com/content/52/6/1801586
http://www.ncbi.nlm.nih.gov/pubmed/30309975?tool=bestpractice.com
[88]Singh D, Papi A, Corradi M, et al. Single inhaler triple therapy versus inhaled corticosteroid plus long-acting beta2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trial. Lancet. 2016 Sep 3;388(10048):963-73.
http://www.ncbi.nlm.nih.gov/pubmed/27598678?tool=bestpractice.com
[89]Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet. 2017 May 13;389(10082):1919-29.
http://www.ncbi.nlm.nih.gov/pubmed/28385353?tool=bestpractice.com
[90]Papi A, Vestbo J, Fabbri L, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet. 2018 Mar 17;391(10125):1076-84.
http://www.ncbi.nlm.nih.gov/pubmed/29429593?tool=bestpractice.com
[91]Lipson DA, Barnacle H, Birk R, et al. FULFIL Trial: once-daily triple therapy for patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017 Aug 15;196(4):438-46.
https://www.atsjournals.org/doi/full/10.1164/rccm.201703-0449OC
http://www.ncbi.nlm.nih.gov/pubmed/28375647?tool=bestpractice.com
[92]Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018 May 3;378(18):1671-80.
https://www.nejm.org/doi/10.1056/NEJMoa1713901
http://www.ncbi.nlm.nih.gov/pubmed/29668352?tool=bestpractice.com
[93]Rojas-Reyes MX, García Morales OM, Dennis RJ, et al. Combination inhaled steroid and long-acting beta₂-agonist in addition to tiotropium versus tiotropium or combination alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2016 Jun 6;(6):CD008532.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD008532.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/27271056?tool=bestpractice.com
[94]Lai CC, Chen CH, Lin CYH, et al. The effects of single inhaler triple therapy vs single inhaler dual therapy or separate triple therapy for the management of chronic obstructive pulmonary disease: a systematic review and meta-analysis of randomized controlled trials. Int J Chron Obstruct Pulmon Dis. 2019 Jul 11;14:1539-48. [Erratum in: Int J Chron Obstruct Pulmon Dis. 2020 Jan 21;15:155-6.]
https://www.dovepress.com/the-effects-of-single-inhaler-triple-therapy-vs-single-inhaler-dual-th-peer-reviewed-fulltext-article-COPD
http://www.ncbi.nlm.nih.gov/pubmed/31371939?tool=bestpractice.com
[95]Rabe KF, Martinez FJ, Ferguson GT, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020 Jul 2;383(1):35-48.
http://www.ncbi.nlm.nih.gov/pubmed/32579807?tool=bestpractice.com
American Thoracic Society guidelines recommend the use of triple therapy in patients who have had one or more exacerbations requiring oral corticosteroids, antibiotics, or hospitalization in the past year and who have symptoms of dyspnea or reduced exercise tolerance despite LABA/LAMA dual therapy.[96]Nici L, Mammen MJ, Charbek E, et al. Pharmacologic management of chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020 May 1;201(9):e56-69.
https://www.atsjournals.org/doi/10.1164/rccm.202003-0625ST
http://www.ncbi.nlm.nih.gov/pubmed/32283960?tool=bestpractice.com
UK guidelines recommend the use of triple therapy in patients who have an exacerbation requiring hospitalization, or two moderate exacerbations within a year, despite dual therapy with LABA/LAMA.[2]National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management. Jul 2019 [internet publication].
https://www.nice.org.uk/guidance/ng115
Patients who take a LABA/LAMA and whose blood eosinophils are <100 cells/microliter should add roflumilast or azithromycin.
LABA/ICS is not recommended by GOLD. However, a patient with COPD who previously had exacerbations that responded to LABA/ICS (and has no current exacerbations) may continue on LABA/ICS; high symptom load may merit escalation to LABA/LAMA/ICS in this patient population. If a patient currently receiving LABA/ICS has no relevant exacerbation history, consider switching to LABA/LAMA. Patients on LABA/ICS with current exacerbations and blood eosinophil count <100 cells/microliter may be candidates for changing to LABA/LAMA. Those with current exacerbations and blood eosinophil count ≥100 cells/microliter while using LABA/ICS should be escalated to triple therapy by addition of a LAMA.
Patients who take LABA/LAMA/ICS may add roflumilast, azithromycin, or dupilumab (an interleukin [IL]-4 receptor antagonist monoclonal antibody). Roflumilast may be considered in patients with forced expiratory volume in 1 second (FEV₁) <50% predicted and chronic bronchitis, particularly if they have had at least one hospitalization for an exacerbation in the last year. The risk of developing antibiotic-resistant organisms should be considered when prescribing azithromycin. Dupilumab may be considered in patients with chronic bronchitis whose blood eosinophil count is ≥300 cells/microliter. ICS can be discontinued if it is ineffective or causing adverse effects. Patients with blood eosinophils ≥300 cells/microliter are at greatest risk of exacerbations after withdrawing ICS.[81]Chapman KR, Hurst JR, Frent SM, et al. Long-term triple therapy de-escalation to indacaterol/glycopyrronium in patients with chronic obstructive pulmonary disease (SUNSET): a randomized, double-blind, triple-dummy clinical trial. Am J Respir Crit Care Med. 2018 Aug 1;198(3):329-39.
https://www.atsjournals.org/doi/10.1164/rccm.201803-0405OC
http://www.ncbi.nlm.nih.gov/pubmed/29779416?tool=bestpractice.com
All patients are candidates for education, vaccination, and smoking cessation interventions.
[Figure caption and citation for the preceding image starts]: Escalation therapy for patients with COPD. Definition of abbreviations: ICS: inhaled corticosteroid; LABA: long-acting beta-2 agonist; LAMA: long-acting muscarinic antagonistGlobal Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2025 report); reproduced with permission [Citation ends].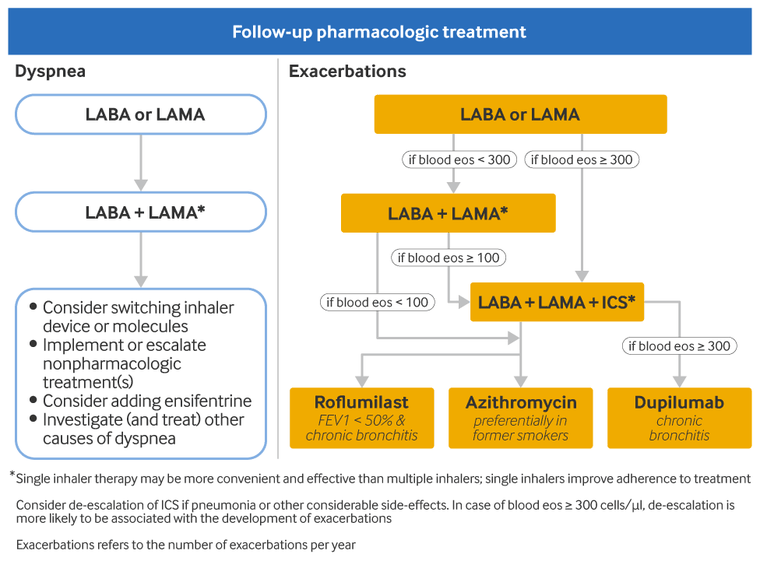
Bronchodilators
Beta-2 agonists are widely used in the treatment of COPD. They increase intracellular cAMP, leading to respiratory smooth muscle relaxation and reduced airway resistance. Muscarinic antagonists (anticholinergics) act as bronchodilators by blocking the cholinergic receptors on the respiratory smooth muscle. This causes muscle relaxation and reduces airflow limitation. Beta agonists and muscarinic antagonists, therefore, provide bronchodilator effects through different pathways. Both are available as short-acting and long-acting preparations.
Short-acting beta-2 agonists (e.g., albuterol, levalbuterol) and short-acting muscarinic antagonists (e.g., ipratropium) improve lung function and breathlessness and quality of life. Ipratropium may have a small benefit over short-acting beta-2 agonists in improving health-related quality of life.[97]Appleton S, Jones T, Poole P, et al. Ipratropium bromide versus short acting beta-2 agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006 Apr 19;(2):CD001387.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD001387.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/16625543?tool=bestpractice.com
These agents can be used as rescue therapy when the patient is using long-acting bronchodilator therapy and may be used as initial treatment for patients in GOLD group A if patients only have occasional dyspnea.[1]Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2025 report. 2025 [internet publication].
https://goldcopd.org/2025-gold-report
[98]Chen AM, Bollmeier SG, Finnegan PM, et al. Long-acting bronchodilator therapy for the treatment of chronic obstructive pulmonary disease. Ann Pharmacother. 2008 Dec;42(12):1832-42.
http://www.ncbi.nlm.nih.gov/pubmed/18957624?tool=bestpractice.com
However, regular use of short-acting bronchodilators is not generally recommended.[1]Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2025 report. 2025 [internet publication].
https://goldcopd.org/2025-gold-report
Tiotropium, a LAMA, has been shown to reduce risk of exacerbation versus placebo or other maintenance treatments.[99]Halpin DM, Vogelmeier C, Pieper MP, et al. Effect of tiotropium on COPD exacerbations: a systematic review. Respir Med. 2016 May;114:1-8.
https://www.resmedjournal.com/article/S0954-6111(16)30030-0/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/27109805?tool=bestpractice.com
[  ]
How does tiotropium compare with ipratropium bromide for people with chronic obstructive pulmonary disease (COPD)?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.2154/fullShow me the answer Newer LAMAs, such as aclidinium, glycopyrrolate, and umeclidinium, have at least comparable efficacy to tiotropium, in terms of change from baseline in trough FEV₁, transitional dyspnea index focal score, St George's Respiratory Questionnaire score, and rescue medication use.[100]Ismaila AS, Huisman EL, Punekar YS, et al. Comparative efficacy of long-acting muscarinic antagonist monotherapies in COPD: a systematic review and network meta-analysis. Int J Chron Obstruct Pulmon Dis. 2015 Nov 16;10(1):2495-517.
https://www.dovepress.com/comparative-efficacy-of-long-acting-muscarinic-antagonist-monotherapie-peer-reviewed-fulltext-article-COPD
http://www.ncbi.nlm.nih.gov/pubmed/26604738?tool=bestpractice.com
Revefenacin is a nebulized LAMA approved for the maintenance treatment of moderate to severe COPD. There is a suggestion of increased cardiovascular-related mortality in some studies of patients taking short-acting muscarinic antagonists and in some studies of patients taking LAMAs.[101]Hilleman DE, Malesker MA, Morrow LE, et al. A systematic review of the cardiovascular risk of inhaled anticholinergics in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2009 Jun 29;4:253-63.
https://www.dovepress.com/getfile.php?fileID=5012
http://www.ncbi.nlm.nih.gov/pubmed/19657399?tool=bestpractice.com
[102]Wang MT, Liou JT, Lin CW, et al. Association of cardiovascular risk with inhaled long-acting bronchodilators in patients with chronic obstructive pulmonary disease: a nested case-control study. JAMA Intern Med. 2018 Feb 1;178(2):229-38.
https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2666790
http://www.ncbi.nlm.nih.gov/pubmed/29297057?tool=bestpractice.com
One study concluded that aclidinium was not associated with an increase in major adverse cardiovascular events, compared with placebo.[103]Wise RA, Chapman KR, Scirica BM, et al. Effect of aclidinium bromide on major cardiovascularevents and exacerbations in high-risk patients with chronic obstructive pulmonary disease: the ASCENT-COPD randomized clinical trial. JAMA. 2019 May 7;321(17):1693-701.
https://jamanetwork.com/journals/jama/fullarticle/2732574
http://www.ncbi.nlm.nih.gov/pubmed/31063575?tool=bestpractice.com
A population-based cohort study found that older men with COPD newly started on LAMAs are at increased risk of urinary tract infections.[104]Gershon AS, Newman AM, Fischer HD, et al. Inhaled long-acting anticholinergics and urinary tract infection in individuals with COPD. COPD. 2017 Feb;14(1):105-12.
http://www.ncbi.nlm.nih.gov/pubmed/27732117?tool=bestpractice.com
]
How does tiotropium compare with ipratropium bromide for people with chronic obstructive pulmonary disease (COPD)?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.2154/fullShow me the answer Newer LAMAs, such as aclidinium, glycopyrrolate, and umeclidinium, have at least comparable efficacy to tiotropium, in terms of change from baseline in trough FEV₁, transitional dyspnea index focal score, St George's Respiratory Questionnaire score, and rescue medication use.[100]Ismaila AS, Huisman EL, Punekar YS, et al. Comparative efficacy of long-acting muscarinic antagonist monotherapies in COPD: a systematic review and network meta-analysis. Int J Chron Obstruct Pulmon Dis. 2015 Nov 16;10(1):2495-517.
https://www.dovepress.com/comparative-efficacy-of-long-acting-muscarinic-antagonist-monotherapie-peer-reviewed-fulltext-article-COPD
http://www.ncbi.nlm.nih.gov/pubmed/26604738?tool=bestpractice.com
Revefenacin is a nebulized LAMA approved for the maintenance treatment of moderate to severe COPD. There is a suggestion of increased cardiovascular-related mortality in some studies of patients taking short-acting muscarinic antagonists and in some studies of patients taking LAMAs.[101]Hilleman DE, Malesker MA, Morrow LE, et al. A systematic review of the cardiovascular risk of inhaled anticholinergics in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2009 Jun 29;4:253-63.
https://www.dovepress.com/getfile.php?fileID=5012
http://www.ncbi.nlm.nih.gov/pubmed/19657399?tool=bestpractice.com
[102]Wang MT, Liou JT, Lin CW, et al. Association of cardiovascular risk with inhaled long-acting bronchodilators in patients with chronic obstructive pulmonary disease: a nested case-control study. JAMA Intern Med. 2018 Feb 1;178(2):229-38.
https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2666790
http://www.ncbi.nlm.nih.gov/pubmed/29297057?tool=bestpractice.com
One study concluded that aclidinium was not associated with an increase in major adverse cardiovascular events, compared with placebo.[103]Wise RA, Chapman KR, Scirica BM, et al. Effect of aclidinium bromide on major cardiovascularevents and exacerbations in high-risk patients with chronic obstructive pulmonary disease: the ASCENT-COPD randomized clinical trial. JAMA. 2019 May 7;321(17):1693-701.
https://jamanetwork.com/journals/jama/fullarticle/2732574
http://www.ncbi.nlm.nih.gov/pubmed/31063575?tool=bestpractice.com
A population-based cohort study found that older men with COPD newly started on LAMAs are at increased risk of urinary tract infections.[104]Gershon AS, Newman AM, Fischer HD, et al. Inhaled long-acting anticholinergics and urinary tract infection in individuals with COPD. COPD. 2017 Feb;14(1):105-12.
http://www.ncbi.nlm.nih.gov/pubmed/27732117?tool=bestpractice.com
LABAs and LAMAs both significantly improve lung function, dyspnea, and health status and reduce exacerbation rates.
[  ]
How does umeclidinium bromide compare with placebo for people with chronic obstructive pulmonary disease (COPD)?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1829/fullShow me the answer[Evidence A]1234286c-1c75-42a9-8a81-2f365d1602afccaAHow does umeclidinium compare with placebo for people with chronic obstructive pulmonary disease (COPD)? In cases of stable COPD, if the decision is made to use single-agent therapy, LAMA may be superior to LABA agents.[105]Rabe KF, Timmer W, Sagkriotis A, et al. Comparison of a combination of tiotropium plus formoterol to salmeterol plus fluticasone in moderate COPD. Chest. 2008 Aug;134(2):255-62.
http://www.ncbi.nlm.nih.gov/pubmed/18403672?tool=bestpractice.com
LAMAs have a greater effect on exacerbation reduction than LABAs in patients with moderate to very severe COPD.[106]Vogelmeier C, Hederer B, Glaab T, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011 Mar 24;364(12):1093-103.
https://www.nejm.org/doi/full/10.1056/NEJMoa1008378
http://www.ncbi.nlm.nih.gov/pubmed/21428765?tool=bestpractice.com
[107]Decramer ML, Chapman KR, Dahl R, et al; INVIGORATE investigators. Once-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): a randomised, blinded, parallel-group study. Lancet Respir Med. 2013 Sep;1(7):524-33.
http://www.ncbi.nlm.nih.gov/pubmed/24461613?tool=bestpractice.com
The long-term safety of LAMA was demonstrated in the UPLIFT trial.[108]Celli B, Decramer M, Kesten S, et al. UPLIFT Study Investigators. Mortality in the 4-year trial of tiotropium (UPLIFT) in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009 Nov 15;180(10):948-55.
https://www.atsjournals.org/doi/full/10.1164/rccm.200906-0876OC
http://www.ncbi.nlm.nih.gov/pubmed/19729663?tool=bestpractice.com
]
How does umeclidinium bromide compare with placebo for people with chronic obstructive pulmonary disease (COPD)?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1829/fullShow me the answer[Evidence A]1234286c-1c75-42a9-8a81-2f365d1602afccaAHow does umeclidinium compare with placebo for people with chronic obstructive pulmonary disease (COPD)? In cases of stable COPD, if the decision is made to use single-agent therapy, LAMA may be superior to LABA agents.[105]Rabe KF, Timmer W, Sagkriotis A, et al. Comparison of a combination of tiotropium plus formoterol to salmeterol plus fluticasone in moderate COPD. Chest. 2008 Aug;134(2):255-62.
http://www.ncbi.nlm.nih.gov/pubmed/18403672?tool=bestpractice.com
LAMAs have a greater effect on exacerbation reduction than LABAs in patients with moderate to very severe COPD.[106]Vogelmeier C, Hederer B, Glaab T, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011 Mar 24;364(12):1093-103.
https://www.nejm.org/doi/full/10.1056/NEJMoa1008378
http://www.ncbi.nlm.nih.gov/pubmed/21428765?tool=bestpractice.com
[107]Decramer ML, Chapman KR, Dahl R, et al; INVIGORATE investigators. Once-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): a randomised, blinded, parallel-group study. Lancet Respir Med. 2013 Sep;1(7):524-33.
http://www.ncbi.nlm.nih.gov/pubmed/24461613?tool=bestpractice.com
The long-term safety of LAMA was demonstrated in the UPLIFT trial.[108]Celli B, Decramer M, Kesten S, et al. UPLIFT Study Investigators. Mortality in the 4-year trial of tiotropium (UPLIFT) in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009 Nov 15;180(10):948-55.
https://www.atsjournals.org/doi/full/10.1164/rccm.200906-0876OC
http://www.ncbi.nlm.nih.gov/pubmed/19729663?tool=bestpractice.com
A LABA/LAMA combination may provide a better therapeutic effect without increasing the adverse effects of each class.[105]Rabe KF, Timmer W, Sagkriotis A, et al. Comparison of a combination of tiotropium plus formoterol to salmeterol plus fluticasone in moderate COPD. Chest. 2008 Aug;134(2):255-62.
http://www.ncbi.nlm.nih.gov/pubmed/18403672?tool=bestpractice.com
[109]Tashkin DP, Littner M, Andrews CP, et al. Concomitant treatment with nebulized formoterol and tiotropium in subjects with COPD: a placebo-controlled trial. Respir Med. 2008 Apr;102(4):479-87.
http://www.ncbi.nlm.nih.gov/pubmed/18258423?tool=bestpractice.com
[110]Tashkin DP, Pearle J, Iezzoni D, et al. Formoterol and tiotropium compared with tiotropium alone for treatment of COPD. COPD. 2009 Feb;6(1):17-25.
http://www.ncbi.nlm.nih.gov/pubmed/19229704?tool=bestpractice.com
[111]Vogelmeier C, Kardos P, Harari S, et al. Formoterol mono- and combination therapy with tiotropium in patients with COPD: a 6-month study. Respir Med. 2008 Nov;102(11):1511-20.
http://www.ncbi.nlm.nih.gov/pubmed/18804362?tool=bestpractice.com
[112]Maltais F, Bjermer L, Kerwin EM, et al. Efficacy of umeclidinium/vilanterol versus umeclidinium and salmeterol monotherapies in symptomatic patients with COPD not receiving inhaled corticosteroids: the EMAX randomised trial. Respir Res. 2019 Oct 30;20(1):238.
https://respiratory-research.biomedcentral.com/articles/10.1186/s12931-019-1193-9
http://www.ncbi.nlm.nih.gov/pubmed/31666084?tool=bestpractice.com
Umeclidinium/vilanterol, glycopyrrolate/formoterol, tiotropium/olodaterol, and aclidinium/formoterol are approved for use in COPD. Systematic reviews and meta-analyses have found that combination therapy with a LABA/LAMA:
reduces exacerbation rate compared with monotherapy
is associated with a clinically significant improvement in lung function and health-related quality of life in patients with mild/moderate COPD, compared with placebo;[113]Maqsood U, Ho TN, Palmer K, et al. Once daily long-acting beta2-agonists and long-acting muscarinic antagonists in a combined inhaler versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2019 Mar 6;(3):CD012930.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD012930.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/30839102?tool=bestpractice.com
[  ]
How does a combined inhaler with once‐daily long‐acting beta2‐agonist (LABA) plus a long‐acting muscarinic antagonist (LAMA) compare with placebo for adults with chronic obstructive pulmonary disease (COPD)?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2551/fullShow me the answer
]
How does a combined inhaler with once‐daily long‐acting beta2‐agonist (LABA) plus a long‐acting muscarinic antagonist (LAMA) compare with placebo for adults with chronic obstructive pulmonary disease (COPD)?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2551/fullShow me the answer
improves FEV₁ and modestly reduces risk of pneumonia in patients with stable chronic obstructive pulmonary disease, but increases odds of all-cause death from 1% to 1.4%.[114]Fukuda N, Horita N, Kaneko A, et al. Long-acting muscarinic antagonist (LAMA) plus long-acting beta-agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2023 Jun 5;6(6):CD012066.
https://www.doi.org/10.1002/14651858.CD012066.pub3
http://www.ncbi.nlm.nih.gov/pubmed/37276335?tool=bestpractice.com
[  ]
How does long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) compare with LABA plus inhaled corticosteroid (ICS) for people with stable chronic obstructive pulmonary disease (COPD)?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.4356/fullShow me the answer
]
How does long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) compare with LABA plus inhaled corticosteroid (ICS) for people with stable chronic obstructive pulmonary disease (COPD)?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.4356/fullShow me the answer
Umeclidinium/vilanterol decreases the risk of exacerbations in patients with mild/moderate COPD.[113]Maqsood U, Ho TN, Palmer K, et al. Once daily long-acting beta2-agonists and long-acting muscarinic antagonists in a combined inhaler versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2019 Mar 6;(3):CD012930.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD012930.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/30839102?tool=bestpractice.com
[  ]
How does a combined inhaler with once‐daily long‐acting beta2‐agonist (LABA) plus a long‐acting muscarinic antagonist (LAMA) compare with placebo for adults with chronic obstructive pulmonary disease (COPD)?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2551/fullShow me the answer One systematic review and network meta-analysis found that all LABA/LAMA fixed-dose combinations had a similar efficacy and safety.[115]Schlueter M, Gonzalez-Rojas N, Baldwin M, et al. Comparative efficacy of fixed-dose combinations of long-acting muscarinic antagonists and long-acting beta2-agonists: a systematic review and network meta-analysis. Ther Adv Respir Dis. 2016 Apr;10(2):89-104.
https://journals.sagepub.com/doi/10.1177/1753465815624612
http://www.ncbi.nlm.nih.gov/pubmed/26746383?tool=bestpractice.com
]
How does a combined inhaler with once‐daily long‐acting beta2‐agonist (LABA) plus a long‐acting muscarinic antagonist (LAMA) compare with placebo for adults with chronic obstructive pulmonary disease (COPD)?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2551/fullShow me the answer One systematic review and network meta-analysis found that all LABA/LAMA fixed-dose combinations had a similar efficacy and safety.[115]Schlueter M, Gonzalez-Rojas N, Baldwin M, et al. Comparative efficacy of fixed-dose combinations of long-acting muscarinic antagonists and long-acting beta2-agonists: a systematic review and network meta-analysis. Ther Adv Respir Dis. 2016 Apr;10(2):89-104.
https://journals.sagepub.com/doi/10.1177/1753465815624612
http://www.ncbi.nlm.nih.gov/pubmed/26746383?tool=bestpractice.com
As outlined above, GOLD makes recommendations on the initial agent based on the patient's risk group (A, B, or E).[1]Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2025 report. 2025 [internet publication].
https://goldcopd.org/2025-gold-report
American Thoracic Society guidelines recommend initiating LABA/LAMA dual therapy in preference to monotherapy in patients with COPD who have dyspnea or exercise intolerance.[96]Nici L, Mammen MJ, Charbek E, et al. Pharmacologic management of chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020 May 1;201(9):e56-69.
https://www.atsjournals.org/doi/10.1164/rccm.202003-0625ST
http://www.ncbi.nlm.nih.gov/pubmed/32283960?tool=bestpractice.com
UK guidelines recommend initiating dual therapy with a LABA/LAMA or LABA/ICS if a patient has symptoms or exacerbations despite nonpharmacologic treatment and using a short-acting bronchodilator as needed. The choice of initial drug regimen in the UK guidance is based on whether or not the patient has features of asthma or features suggesting corticosteroid responsiveness.[2]National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management. Jul 2019 [internet publication].
https://www.nice.org.uk/guidance/ng115
Inhaled corticosteroids (ICS)
When indicated in patients with COPD, ICS should always be prescribed in combination with long-acting bronchodilators. ICS are believed to be effective because of their anti-inflammatory effects. Long-term ICS use reduces the need to use rescue therapy and reduces exacerbations, and may also decrease mortality.[116]Spencer S, Calverley PM, Burge PS, et al. Impact of preventing exacerbations on deterioration of health status in COPD. Eur Respir J. 2004 May;23(5):698-702.
https://erj.ersjournals.com/content/23/5/698
http://www.ncbi.nlm.nih.gov/pubmed/15176682?tool=bestpractice.com
[117]Sin DD, Wu L, Anderson JA, et al. Inhaled corticosteroids and mortality in chronic obstructive pulmonary disease. Thorax. 2005 Dec;60(12):992-7.
http://www.ncbi.nlm.nih.gov/pubmed/16227327?tool=bestpractice.com
[118]Lee HW, Park J, Jo J, et al. Comparisons of exacerbations and mortality among regular inhaled therapies for patients with stable chronic obstructive pulmonary disease: systematic review and Bayesian network meta-analysis. PLoS Med. 2019 Nov;16(11):e1002958.
https://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1002958
http://www.ncbi.nlm.nih.gov/pubmed/31730642?tool=bestpractice.com
The effect of treatment regimens containing ICS is higher in patients at higher risk of exacerbations (two or more exacerbations and/or one hospitalization for an exacerbation in the previous year).[90]Papi A, Vestbo J, Fabbri L, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet. 2018 Mar 17;391(10125):1076-84.
http://www.ncbi.nlm.nih.gov/pubmed/29429593?tool=bestpractice.com
[92]Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018 May 3;378(18):1671-80.
https://www.nejm.org/doi/10.1056/NEJMoa1713901
http://www.ncbi.nlm.nih.gov/pubmed/29668352?tool=bestpractice.com
[119]Wedzicha JA, Banerji D, Chapman KR, et al; FLAME Investigators. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med. 2016 Jun 9;374(23):2222-34.
https://www.nejm.org/doi/10.1056/NEJMoa1516385
http://www.ncbi.nlm.nih.gov/pubmed/27181606?tool=bestpractice.com
Blood eosinophil count may predict the effectiveness of adding ICS to regular long-acting bronchodilator treatment to prevent exacerbations.[77]Harries TH, Rowland V, Corrigan CJ, et al. Blood eosinophil count, a marker of inhaled corticosteroid effectiveness in preventing COPD exacerbations in post-hoc RCT and observational studies: systematic review and meta-analysis. Respir Res. 2020 Jan 3;21(1):3.
https://respiratory-research.biomedcentral.com/articles/10.1186/s12931-019-1268-7
http://www.ncbi.nlm.nih.gov/pubmed/31900184?tool=bestpractice.com
[78]Oshagbemi OA, Odiba JO, Daniel A, et al. Absolute blood eosinophil counts to guide inhaled corticosteroids therapy among patients with COPD: systematic review and meta-analysis. Curr Drug Targets. 2019;20(16):1670-9.
http://www.ncbi.nlm.nih.gov/pubmed/31393244?tool=bestpractice.com
[79]Pascoe S, Barnes N, Brusselle G, et al. Blood eosinophils and treatment response with triple and dual combination therapy in chronic obstructive pulmonary disease: analysis of the IMPACT trial. Lancet Respir Med. 2019 Sep;7(9):745-56.
http://www.ncbi.nlm.nih.gov/pubmed/31281061?tool=bestpractice.com
Little or no effect is seen at blood eosinophil counts of <100 cells/microliter, while maximal effect is seen at blood eosinophil counts of ≥300 cells/microliter.[76]Bafadhel M, Peterson S, De Blas MA, et al. Predictors of exacerbation risk and response to budesonide in patients with chronic obstructive pulmonary disease: a post-hoc analysis of three randomised trials. Lancet Respir Med. 2018 Feb;6(2):117-26.
http://www.ncbi.nlm.nih.gov/pubmed/29331313?tool=bestpractice.com
[80]Cazzola M, Rogliani P, Calzetta L, et al. Triple therapy versus single and dual long-acting bronchodilator therapy in COPD: a systematic review and meta-analysis. Eur Respir J. 2018 Dec 13;52(6):1801586.
https://erj.ersjournals.com/content/52/6/1801586
http://www.ncbi.nlm.nih.gov/pubmed/30309975?tool=bestpractice.com
These thresholds indicate approximate cut-off values that may help clinicians predict the likelihood of a treatment benefit.[1]Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2025 report. 2025 [internet publication].
https://goldcopd.org/2025-gold-report
Former smokers are more corticosteroid-responsive than current smokers at any eosinophil count.[79]Pascoe S, Barnes N, Brusselle G, et al. Blood eosinophils and treatment response with triple and dual combination therapy in chronic obstructive pulmonary disease: analysis of the IMPACT trial. Lancet Respir Med. 2019 Sep;7(9):745-56.
http://www.ncbi.nlm.nih.gov/pubmed/31281061?tool=bestpractice.com
Both current and former smokers with COPD can benefit from ICS in terms of lung function and rates of exacerbations, although the effect is smaller for heavy or current smokers compared with light or former smokers.[92]Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018 May 3;378(18):1671-80.
https://www.nejm.org/doi/10.1056/NEJMoa1713901
http://www.ncbi.nlm.nih.gov/pubmed/29668352?tool=bestpractice.com
[120]Sonnex K, Alleemudder H, Knaggs R. Impact of smoking status on the efficacy of inhaled corticosteroids in chronic obstructive pulmonary disease: a systematic review. BMJ Open. 2020 Apr 15;10(4):e037509.
https://bmjopen.bmj.com/content/10/4/e037509
http://www.ncbi.nlm.nih.gov/pubmed/32300001?tool=bestpractice.com
Short-term ICS use (≤1 year) may be associated with greater improvements in FEV₁ than long-term use, although further studies are needed to better understand the effect of treatment on lung function.[121]Whittaker HR, Jarvis D, Sheikh MR, et al. Inhaled corticosteroids and FEV1 decline in chronic obstructive pulmonary disease: a systematic review. Respir Res. 2019 Dec 4;20(1):277.
https://respiratory-research.biomedcentral.com/articles/10.1186/s12931-019-1249-x
http://www.ncbi.nlm.nih.gov/pubmed/31801539?tool=bestpractice.com
Several studies have pointed to an increased risk of pneumonia in patients with COPD taking ICS.[85]Yang IA, Ferry OR, Clarke MS, et al. Inhaled corticosteroids versus placebo for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2023 Mar 27;(3):CD002991.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD002991.pub4/full
http://www.ncbi.nlm.nih.gov/pubmed/36971693?tool=bestpractice.com
[  ]
What are the benefits and harms of inhaled corticosteroids (ICS) in people with stable chronic obstructive pulmonary disease (COPD)?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.4293/fullShow me the answer This risk is higher for fluticasone in comparison with budesonide.[122]Suissa S, Patenaude V, Lapi F, et al. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax. 2013 Nov;68(11):1029-36.
https://thorax.bmj.com/content/68/11/1029.long
http://www.ncbi.nlm.nih.gov/pubmed/24130228?tool=bestpractice.com
[123]Lodise TP, Li J, Gandhi HN, et al. Intraclass difference in pneumonia risk with fluticasone and budesonide in COPD: a systematic review of evidence from direct-comparison studies. Int J Chron Obstruct Pulmon Dis. 2020 Nov 11;15:2889-900.
https://www.dovepress.com/intraclass-difference-in-pneumonia-risk-with-fluticasone-and-budesonid-peer-reviewed-fulltext-article-COPD
http://www.ncbi.nlm.nih.gov/pubmed/33204085?tool=bestpractice.com
[124]Yang M, Du Y, Chen H, et al. Inhaled corticosteroids and risk of pneumonia in patients with chronic obstructive pulmonary disease: a meta-analysis of randomized controlled trials. Int Immunopharmacol. 2019 Dec;77:105950.
http://www.ncbi.nlm.nih.gov/pubmed/31629940?tool=bestpractice.com
A study in a large cohort of Danish patients found the risk of acquiring Pseudomonas aeruginosa, a common cause of hospital-acquired pneumonia, to be dose-dependent, with high-dose ICS associated with the greatest risk. The study also found that patients with P aeruginosa were more likely to have a lower BMI and FEV₁ than P aeruginosa-negative patients.[125]Eklöf J, Ingebrigtsen TS, Sørensen R, et al. Use of inhaled corticosteroids and risk of acquiring Pseudomonas aeruginosa in patients with chronic obstructive pulmonary disease. Thorax. 2022 Jun;77(6):573-80.
https://thorax.bmj.com/content/77/6/573
http://www.ncbi.nlm.nih.gov/pubmed/34446524?tool=bestpractice.com
One systematic review and meta-analysis found that, despite a significant increase in unadjusted risk of pneumonia associated with use of ICS, pneumonia fatality and overall mortality were not increased in randomized controlled trials and were decreased in observational studies.[126]Festic E, Bansal V, Gupta E, et al. Association of inhaled corticosteroids with incident pneumonia and mortality in COPD patients; systematic review and meta-analysis. COPD. 2016 Jun;13(3):312-26.
https://www.tandfonline.com/doi/full/10.3109/15412555.2015.1081162
http://www.ncbi.nlm.nih.gov/pubmed/26645797?tool=bestpractice.com
Therefore, an individualized treatment approach that assesses a patient's risk of pneumonia versus the benefit of decreased exacerbations should be implemented.[85]Yang IA, Ferry OR, Clarke MS, et al. Inhaled corticosteroids versus placebo for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2023 Mar 27;(3):CD002991.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD002991.pub4/full
http://www.ncbi.nlm.nih.gov/pubmed/36971693?tool=bestpractice.com
[127]Welte T. Inhaled corticosteroids in COPD and the risk of pneumonia. Lancet. 2009 Aug 29;374(9691):668-70.
http://www.ncbi.nlm.nih.gov/pubmed/19716946?tool=bestpractice.com
[128]Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014 Mar 10;(3):CD010115.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD010115.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/24615270?tool=bestpractice.com
Concern is also raised with regards to increased risk of tuberculosis and influenza in adult patients with COPD who are on ICS therapy, although one meta-analysis found that less than 1% of all assessed tuberculosis cases were attributable to ICS exposure.[129]Dong YH, Chang CH, Lin Wu FL, et al. Use of inhaled corticosteroids in patients with COPD and the risk of TB and influenza: a systematic review and meta-analysis of randomized controlled trials. Chest. 2014 Jun;145(6):1286-97.
http://www.ncbi.nlm.nih.gov/pubmed/24504044?tool=bestpractice.com
[130]Castellana G, Castellana M, Castellana C, et al. Inhaled corticosteroids and risk of tuberculosis in patients with obstructive lung diseases: a systematic review and meta-analysis of non-randomized studies. Int J Chron Obstruct Pulmon Dis. 2019 Sep 26;14:2219-27.
https://www.dovepress.com/inhaled-corticosteroids-and-risk-of-tuberculosis-in-patients-with-obst-peer-reviewed-fulltext-article-COPD
http://www.ncbi.nlm.nih.gov/pubmed/31576118?tool=bestpractice.com
ICS may also cause oropharyngeal candidiasis and hoarseness.[85]Yang IA, Ferry OR, Clarke MS, et al. Inhaled corticosteroids versus placebo for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2023 Mar 27;(3):CD002991.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD002991.pub4/full
http://www.ncbi.nlm.nih.gov/pubmed/36971693?tool=bestpractice.com
[
]
What are the benefits and harms of inhaled corticosteroids (ICS) in people with stable chronic obstructive pulmonary disease (COPD)?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.4293/fullShow me the answer This risk is higher for fluticasone in comparison with budesonide.[122]Suissa S, Patenaude V, Lapi F, et al. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax. 2013 Nov;68(11):1029-36.
https://thorax.bmj.com/content/68/11/1029.long
http://www.ncbi.nlm.nih.gov/pubmed/24130228?tool=bestpractice.com
[123]Lodise TP, Li J, Gandhi HN, et al. Intraclass difference in pneumonia risk with fluticasone and budesonide in COPD: a systematic review of evidence from direct-comparison studies. Int J Chron Obstruct Pulmon Dis. 2020 Nov 11;15:2889-900.
https://www.dovepress.com/intraclass-difference-in-pneumonia-risk-with-fluticasone-and-budesonid-peer-reviewed-fulltext-article-COPD
http://www.ncbi.nlm.nih.gov/pubmed/33204085?tool=bestpractice.com
[124]Yang M, Du Y, Chen H, et al. Inhaled corticosteroids and risk of pneumonia in patients with chronic obstructive pulmonary disease: a meta-analysis of randomized controlled trials. Int Immunopharmacol. 2019 Dec;77:105950.
http://www.ncbi.nlm.nih.gov/pubmed/31629940?tool=bestpractice.com
A study in a large cohort of Danish patients found the risk of acquiring Pseudomonas aeruginosa, a common cause of hospital-acquired pneumonia, to be dose-dependent, with high-dose ICS associated with the greatest risk. The study also found that patients with P aeruginosa were more likely to have a lower BMI and FEV₁ than P aeruginosa-negative patients.[125]Eklöf J, Ingebrigtsen TS, Sørensen R, et al. Use of inhaled corticosteroids and risk of acquiring Pseudomonas aeruginosa in patients with chronic obstructive pulmonary disease. Thorax. 2022 Jun;77(6):573-80.
https://thorax.bmj.com/content/77/6/573
http://www.ncbi.nlm.nih.gov/pubmed/34446524?tool=bestpractice.com
One systematic review and meta-analysis found that, despite a significant increase in unadjusted risk of pneumonia associated with use of ICS, pneumonia fatality and overall mortality were not increased in randomized controlled trials and were decreased in observational studies.[126]Festic E, Bansal V, Gupta E, et al. Association of inhaled corticosteroids with incident pneumonia and mortality in COPD patients; systematic review and meta-analysis. COPD. 2016 Jun;13(3):312-26.
https://www.tandfonline.com/doi/full/10.3109/15412555.2015.1081162
http://www.ncbi.nlm.nih.gov/pubmed/26645797?tool=bestpractice.com
Therefore, an individualized treatment approach that assesses a patient's risk of pneumonia versus the benefit of decreased exacerbations should be implemented.[85]Yang IA, Ferry OR, Clarke MS, et al. Inhaled corticosteroids versus placebo for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2023 Mar 27;(3):CD002991.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD002991.pub4/full
http://www.ncbi.nlm.nih.gov/pubmed/36971693?tool=bestpractice.com
[127]Welte T. Inhaled corticosteroids in COPD and the risk of pneumonia. Lancet. 2009 Aug 29;374(9691):668-70.
http://www.ncbi.nlm.nih.gov/pubmed/19716946?tool=bestpractice.com
[128]Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014 Mar 10;(3):CD010115.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD010115.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/24615270?tool=bestpractice.com
Concern is also raised with regards to increased risk of tuberculosis and influenza in adult patients with COPD who are on ICS therapy, although one meta-analysis found that less than 1% of all assessed tuberculosis cases were attributable to ICS exposure.[129]Dong YH, Chang CH, Lin Wu FL, et al. Use of inhaled corticosteroids in patients with COPD and the risk of TB and influenza: a systematic review and meta-analysis of randomized controlled trials. Chest. 2014 Jun;145(6):1286-97.
http://www.ncbi.nlm.nih.gov/pubmed/24504044?tool=bestpractice.com
[130]Castellana G, Castellana M, Castellana C, et al. Inhaled corticosteroids and risk of tuberculosis in patients with obstructive lung diseases: a systematic review and meta-analysis of non-randomized studies. Int J Chron Obstruct Pulmon Dis. 2019 Sep 26;14:2219-27.
https://www.dovepress.com/inhaled-corticosteroids-and-risk-of-tuberculosis-in-patients-with-obst-peer-reviewed-fulltext-article-COPD
http://www.ncbi.nlm.nih.gov/pubmed/31576118?tool=bestpractice.com
ICS may also cause oropharyngeal candidiasis and hoarseness.[85]Yang IA, Ferry OR, Clarke MS, et al. Inhaled corticosteroids versus placebo for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2023 Mar 27;(3):CD002991.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD002991.pub4/full
http://www.ncbi.nlm.nih.gov/pubmed/36971693?tool=bestpractice.com
[  ]
What are the benefits and harms of inhaled corticosteroids (ICS) in people with stable chronic obstructive pulmonary disease (COPD)?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.4293/fullShow me the answer
]
What are the benefits and harms of inhaled corticosteroids (ICS) in people with stable chronic obstructive pulmonary disease (COPD)?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.4293/fullShow me the answer
Although there have been reports of ICS use either increasing or decreasing the risk of lung cancer, the available data do not appear to support either conclusion; further studies are needed.[1]Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2025 report. 2025 [internet publication].
https://goldcopd.org/2025-gold-report
Clinicians should weigh the potential benefits and risks of prescribing ICS and discuss these with the patient.[2]National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management. Jul 2019 [internet publication].
https://www.nice.org.uk/guidance/ng115
A history of hospitalization(s) for exacerbations of COPD, two or more moderate exacerbations per year despite regular long-acting bronchodilators, blood eosinophils of ≥300 cells/microliter, and/or previous or concomitant asthma all strongly favor initiating ICS.[131]Agusti A, Fabbri LM, Singh D, et al. Inhaled corticosteroids in COPD: friend or foe? Eur Respir J. 2018 Dec 13;52(6):1801219.
https://erj.ersjournals.com/content/52/6/1801219
http://www.ncbi.nlm.nih.gov/pubmed/30190269?tool=bestpractice.com
Repeated episodes of pneumonia, blood eosinophils <100 cells/microliter, and/or history of mycobacterial infection are all factors against the use of ICS.[131]Agusti A, Fabbri LM, Singh D, et al. Inhaled corticosteroids in COPD: friend or foe? Eur Respir J. 2018 Dec 13;52(6):1801219.
https://erj.ersjournals.com/content/52/6/1801219
http://www.ncbi.nlm.nih.gov/pubmed/30190269?tool=bestpractice.com
Use of ICS can be considered in patients with one moderate exacerbation of COPD per year despite regular long-acting bronchodilator therapy and/or peripheral eosinophils 100-300 cells/microliter.[131]Agusti A, Fabbri LM, Singh D, et al. Inhaled corticosteroids in COPD: friend or foe? Eur Respir J. 2018 Dec 13;52(6):1801219.
https://erj.ersjournals.com/content/52/6/1801219
http://www.ncbi.nlm.nih.gov/pubmed/30190269?tool=bestpractice.com
The European Respiratory Society has produced a guideline on the withdrawal of inhaled corticosteroids in COPD.[132]Chalmers JD, Laska IF, Franssen FME, et al. Withdrawal of inhaled corticosteroids in COPD: a European Respiratory Society guideline. Eur Respir J. 2020 Jun 4;55(6):2000351.
https://erj.ersjournals.com/content/55/6/2000351
http://www.ncbi.nlm.nih.gov/pubmed/32366483?tool=bestpractice.com
Long-term use of oral corticosteroids in COPD is not recommended.[96]Nici L, Mammen MJ, Charbek E, et al. Pharmacologic management of chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020 May 1;201(9):e56-69.
https://www.atsjournals.org/doi/10.1164/rccm.202003-0625ST
http://www.ncbi.nlm.nih.gov/pubmed/32283960?tool=bestpractice.com
Some patients with severe disease are unable to completely stop treatment after starting oral corticosteroids for an acute exacerbation. In this case, the dose should be kept as low as possible and consideration given to osteoporosis prophylaxis.[2]National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management. Jul 2019 [internet publication].
https://www.nice.org.uk/guidance/ng115
Combined bronchodilator and corticosteroid preparations
A combination preparation of a long-acting bronchodilator and an ICS may be used for patients who require both these agents.
[  ]
What are the effects of long‐acting inhaled therapies for adults with chronic obstructive pulmonary disease (COPD)?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2364/fullShow me the answer This is convenient and may help with compliance in some patients. The choice of therapy in this class is based on availability and individual response and preference.[133]Tricco AC, Strifler L, Veroniki AA, et al. Comparative safety and effectiveness of long-acting inhaled agents for treating chronic obstructive pulmonary disease: a systematic review and network meta-analysis. BMJ Open. 2015 Oct 26;5(10):e009183. [Erratum in: BMJ Open. 2019 May 1;9(4):e009183corr1.]
https://bmjopen.bmj.com/content/5/10/e009183.long
http://www.ncbi.nlm.nih.gov/pubmed/26503392?tool=bestpractice.com
The combination may be provided in separate inhalers or a combination inhaler.
]
What are the effects of long‐acting inhaled therapies for adults with chronic obstructive pulmonary disease (COPD)?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2364/fullShow me the answer This is convenient and may help with compliance in some patients. The choice of therapy in this class is based on availability and individual response and preference.[133]Tricco AC, Strifler L, Veroniki AA, et al. Comparative safety and effectiveness of long-acting inhaled agents for treating chronic obstructive pulmonary disease: a systematic review and network meta-analysis. BMJ Open. 2015 Oct 26;5(10):e009183. [Erratum in: BMJ Open. 2019 May 1;9(4):e009183corr1.]
https://bmjopen.bmj.com/content/5/10/e009183.long
http://www.ncbi.nlm.nih.gov/pubmed/26503392?tool=bestpractice.com
The combination may be provided in separate inhalers or a combination inhaler.
Multiple studies support triple therapy with LABA/LAMA/ICS as being superior to single- or double-agent therapy with LABA/LAMA or LABA/ICS regarding rate of moderate to severe COPD exacerbations and rate of hospitalization.[80]Cazzola M, Rogliani P, Calzetta L, et al. Triple therapy versus single and dual long-acting bronchodilator therapy in COPD: a systematic review and meta-analysis. Eur Respir J. 2018 Dec 13;52(6):1801586.
https://erj.ersjournals.com/content/52/6/1801586
http://www.ncbi.nlm.nih.gov/pubmed/30309975?tool=bestpractice.com
[88]Singh D, Papi A, Corradi M, et al. Single inhaler triple therapy versus inhaled corticosteroid plus long-acting beta2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trial. Lancet. 2016 Sep 3;388(10048):963-73.
http://www.ncbi.nlm.nih.gov/pubmed/27598678?tool=bestpractice.com
[89]Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet. 2017 May 13;389(10082):1919-29.
http://www.ncbi.nlm.nih.gov/pubmed/28385353?tool=bestpractice.com
[90]Papi A, Vestbo J, Fabbri L, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet. 2018 Mar 17;391(10125):1076-84.
http://www.ncbi.nlm.nih.gov/pubmed/29429593?tool=bestpractice.com
[91]Lipson DA, Barnacle H, Birk R, et al. FULFIL Trial: once-daily triple therapy for patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017 Aug 15;196(4):438-46.
https://www.atsjournals.org/doi/full/10.1164/rccm.201703-0449OC
http://www.ncbi.nlm.nih.gov/pubmed/28375647?tool=bestpractice.com
[92]Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018 May 3;378(18):1671-80.
https://www.nejm.org/doi/10.1056/NEJMoa1713901
http://www.ncbi.nlm.nih.gov/pubmed/29668352?tool=bestpractice.com
[93]Rojas-Reyes MX, García Morales OM, Dennis RJ, et al. Combination inhaled steroid and long-acting beta₂-agonist in addition to tiotropium versus tiotropium or combination alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2016 Jun 6;(6):CD008532.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD008532.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/27271056?tool=bestpractice.com
[94]Lai CC, Chen CH, Lin CYH, et al. The effects of single inhaler triple therapy vs single inhaler dual therapy or separate triple therapy for the management of chronic obstructive pulmonary disease: a systematic review and meta-analysis of randomized controlled trials. Int J Chron Obstruct Pulmon Dis. 2019 Jul 11;14:1539-48. [Erratum in: Int J Chron Obstruct Pulmon Dis. 2020 Jan 21;15:155-6.]
https://www.dovepress.com/the-effects-of-single-inhaler-triple-therapy-vs-single-inhaler-dual-th-peer-reviewed-fulltext-article-COPD
http://www.ncbi.nlm.nih.gov/pubmed/31371939?tool=bestpractice.com
[95]Rabe KF, Martinez FJ, Ferguson GT, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020 Jul 2;383(1):35-48.
http://www.ncbi.nlm.nih.gov/pubmed/32579807?tool=bestpractice.com
Use of ICS also slows the rate of decline in lung function following an exacerbation in patients with mild to moderate COPD and elevated blood eosinophils.[134]Kerkhof M, Voorham J, Dorinsky P, et al. Association between COPD exacerbations and lung function decline during maintenance therapy. Thorax. 2020 Sep;75(9):744-53.
https://thorax.bmj.com/content/75/9/744
http://www.ncbi.nlm.nih.gov/pubmed/32532852?tool=bestpractice.com
One randomized controlled trial has reported a reduction in all-cause mortality in patients with FEV₁ <50% and at least one exacerbation in the past year who take fluticasone furoate/umeclidinium/vilanterol, compared with patients taking umeclidinium/vilanterol. Patients with mild COPD and at least two moderate or one severe exacerbations in the last year also had reduced all-cause mortality when taking fluticasone furoate/umeclidinium/vilanterol, compared with umeclidinium/vilanterol.[135]Lipson DA, Crim C, Criner GJ, et al. Reduction in all-cause mortality with fluticasone furoate/umeclidinium/vilanterol in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2020 Jun 15;201(12):1508-16.
https://www.atsjournals.org/doi/10.1164/rccm.201911-2207OC
http://www.ncbi.nlm.nih.gov/pubmed/32162970?tool=bestpractice.com
Another randomized controlled trial had similar findings in terms of mortality in the triple therapy arm (budesonide/glycopyrrolate/formoterol), but only at the higher dose of ICS.[95]Rabe KF, Martinez FJ, Ferguson GT, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020 Jul 2;383(1):35-48.
http://www.ncbi.nlm.nih.gov/pubmed/32579807?tool=bestpractice.com
[136]Martinez FJ, Rabe KF, Ferguson GT, et al. Reduced all-cause mortality in the ETHOS trial of budesonide/glycopyrrolate/formoterol for chronic obstructive pulmonary disease. A randomized, double-blind, multicenter, parallel-group study. Am J Respir Crit Care Med. 2021 Mar 1;203(5):553-64.
https://www.atsjournals.org/doi/10.1164/rccm.202006-2618OC
http://www.ncbi.nlm.nih.gov/pubmed/33252985?tool=bestpractice.com
The same study showed that increasing the dose of budesonide in triple therapy does not decrease the rate of exacerbations, compared with standard dose triple therapy.[95]Rabe KF, Martinez FJ, Ferguson GT, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020 Jul 2;383(1):35-48.
http://www.ncbi.nlm.nih.gov/pubmed/32579807?tool=bestpractice.com
For both studies, there were no differences in mortality compared with LABA/ICS.[95]Rabe KF, Martinez FJ, Ferguson GT, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020 Jul 2;383(1):35-48.
http://www.ncbi.nlm.nih.gov/pubmed/32579807?tool=bestpractice.com
[135]Lipson DA, Crim C, Criner GJ, et al. Reduction in all-cause mortality with fluticasone furoate/umeclidinium/vilanterol in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2020 Jun 15;201(12):1508-16.
https://www.atsjournals.org/doi/10.1164/rccm.201911-2207OC
http://www.ncbi.nlm.nih.gov/pubmed/32162970?tool=bestpractice.com
[136]Martinez FJ, Rabe KF, Ferguson GT, et al. Reduced all-cause mortality in the ETHOS trial of budesonide/glycopyrrolate/formoterol for chronic obstructive pulmonary disease. A randomized, double-blind, multicenter, parallel-group study. Am J Respir Crit Care Med. 2021 Mar 1;203(5):553-64.
https://www.atsjournals.org/doi/10.1164/rccm.202006-2618OC
http://www.ncbi.nlm.nih.gov/pubmed/33252985?tool=bestpractice.com
A post hoc pooled analysis of three trials of triple therapy in patients with COPD and severe airflow limitation and a history of exacerbations showed a nonsignificant trend for lower mortality with triple therapy compared with non-ICS treatments.[137]Vestbo J, Fabbri L, Papi A, et al. Inhaled corticosteroid containing combinations and mortality in COPD. Eur Respir J. 2018 Dec 13;52(6):1801230.
https://erj.ersjournals.com/content/52/6/1801230
http://www.ncbi.nlm.nih.gov/pubmed/30209195?tool=bestpractice.com
These results are strengthened by findings from a meta-analysis of over 200 studies: triple therapy provided a significant reduction in mortality versus dual therapy, although was associated with greater risk of pneumonia. No differences were observed between regimens in lung function or health-related quality of life.[138]Axson EL, Lewis A, Potts J, et al. Inhaled therapies for chronic obstructive pulmonary disease: a systematic review and meta-analysis. BMJ Open. 2020 Sep 29;10(9):e036455.
https://bmjopen.bmj.com/content/10/9/e036455
http://www.ncbi.nlm.nih.gov/pubmed/32994234?tool=bestpractice.com
Before prescribing triple therapy, clinicians should assess whether another physical or mental condition could be causing the patient's symptoms. UK guidelines advise clinicians to review patients taking triple therapy for relief of daily symptoms after 3 months. Treatment should be changed to LABA/LAMA if the patient's symptoms have not improved.[2]National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management. Jul 2019 [internet publication].
https://www.nice.org.uk/guidance/ng115
The ICS may be withdrawn if the patient has had no exacerbations in the past year.[96]Nici L, Mammen MJ, Charbek E, et al. Pharmacologic management of chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020 May 1;201(9):e56-69.
https://www.atsjournals.org/doi/10.1164/rccm.202003-0625ST
http://www.ncbi.nlm.nih.gov/pubmed/32283960?tool=bestpractice.com
One systematic review of data from real-world studies found little to no evidence of worsened outcomes when ICS was withdrawn and followed by appropriate pharmacologic management in patients with moderate to severe COPD.[139]Rogliani P, Ritondo BL, Gabriele M, et al. Optimizing de-escalation of inhaled corticosteroids in COPD: a systematic review of real-world findings. Expert Rev Clin Pharmacol. 2020 Sep;13(9):977-90.
https://www.tandfonline.com/doi/full/10.1080/17512433.2020.1817739
http://www.ncbi.nlm.nih.gov/pubmed/32885691?tool=bestpractice.com
Phosphodiesterase inhibitors
Roflumilast is an oral phosphodiesterase (PDE)-4 inhibitor that inhibits the breakdown of cAMP. It may be considered in patients with FEV₁ <50% predicted and chronic bronchitis who are taking LABA/LAMA/ICS, particularly if they have had at least one hospitalization for an exacerbation in the last year.[1]Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2025 report. 2025 [internet publication].
https://goldcopd.org/2025-gold-report
Roflumilast offers benefit in improving lung function and reducing the likelihood of exacerbations. However, it has little impact on quality of life or symptoms.[140]Janjua S, Fortescue R, Poole P. Phosphodiesterase-4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2020 May 1;(5):CD002309.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD002309.pub6/full
http://www.ncbi.nlm.nih.gov/pubmed/32356609?tool=bestpractice.com
[  ]
How do phosphodiesterase‐4 inhibitors compare with placebo for people with chronic obstructive pulmonary disease?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.3151/fullShow me the answer
]
How do phosphodiesterase‐4 inhibitors compare with placebo for people with chronic obstructive pulmonary disease?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.3151/fullShow me the answer
Ensifentrine is a selective PDE-3 and PDE-4 inhibitor approved in the US for the maintenance treatment of COPD in adults. It combines bronchodilator and nonsteroidal anti-inflammatory effects within a single molecule. Ensifentrine may be considered as an add-on bronchodilator for patients with stable COPD taking LABA/LAMA who have persistent dyspnea symptoms.[1]Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2025 report. 2025 [internet publication].
https://goldcopd.org/2025-gold-report
Clinical benefits were apparent when used as monotherapy and with other maintenance therapies.[141]Anzueto A, Barjaktarevic IZ, Siler TM, et al. Ensifentrine, a novel phosphodiesterase 3 and 4 inhibitor for the treatment of chronic obstructive pulmonary disease: randomized, double-blind, placebo-controlled, multicenter phase III trials (the ENHANCE trials). Am J Respir Crit Care Med. 2023 Aug 15;208(4):406-16.
https://www.atsjournals.org/doi/10.1164/rccm.202306-0944OC?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/37364283?tool=bestpractice.com
Nonetheless, enfensitrine’s place in therapy requires further study.
Antibiotics
Prophylactic antibiotics, such as macrolides, may be considered for reducing the risk of acute exacerbation, particularly in patients who have frequent exacerbations and are refractory to standard therapy.[142]Simoens S, Laekeman G, Decramer M. Preventing COPD exacerbations with macrolides: a review and budget impact analysis. Respir Med. 2013 May;107(5):637-48.
http://www.ncbi.nlm.nih.gov/pubmed/23352223?tool=bestpractice.com
[143]Lee JS, Park DA, Hong Y, et al. Systematic review and meta-analysis of prophylactic antibiotics in COPD and/or chronic bronchitis. Int J Tuberc Lung Dis. 2013 Feb;17(2):153-62.
http://www.ncbi.nlm.nih.gov/pubmed/23317949?tool=bestpractice.com
[144]Uzun S, Djamin RS, Kluytmans JA, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2014 May;2(5):361-8.
http://www.ncbi.nlm.nih.gov/pubmed/24746000?tool=bestpractice.com
[145]Janjua S, Mathioudakis AG, Fortescue R, et al. Prophylactic antibiotics for adults with chronic obstructive pulmonary disease: a network meta-analysis. Cochrane Database Syst Rev. 2021 Jan 15;(1):CD013198.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD013198.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/33448349?tool=bestpractice.com
One Cochrane review ranked macrolides first in reducing exacerbations and serious adverse events, and improving quality of life, above fluoroquinolones and tetracyclines.[145]Janjua S, Mathioudakis AG, Fortescue R, et al. Prophylactic antibiotics for adults with chronic obstructive pulmonary disease: a network meta-analysis. Cochrane Database Syst Rev. 2021 Jan 15;(1):CD013198.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD013198.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/33448349?tool=bestpractice.com
Use of prophylactic macrolide antibiotics decreases the frequency of exacerbations in patients with COPD but long-term azithromycin use is associated with clinically significant hearing loss, which in many cases was reversible.[146]Herath SC, Normansell R, Maisey S, et al. Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2018 Oct 30;(10):CD009764.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD009764.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/30376188?tool=bestpractice.com
[  ]
What are the effects of prophylactic antibiotics for people with chronic obstructive pulmonary disease (COPD)?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2431/fullShow me the answer There are no data showing the efficacy or safety of chronic azithromycin treatment beyond 1 year of treatment.
]
What are the effects of prophylactic antibiotics for people with chronic obstructive pulmonary disease (COPD)?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2431/fullShow me the answer There are no data showing the efficacy or safety of chronic azithromycin treatment beyond 1 year of treatment.
Azithromycin therapy is believed to be most effective in preventing acute exacerbation, with greater efficacy seen in older patients and milder GOLD stages. Little evidence of treatment benefit is seen in current smokers.[147]Han MK, Tayob N, Murray S, et al. Predictors of chronic obstructive pulmonary disease exacerbation reduction in response to daily azithromycin therapy. Am J Respir Crit Care Med. 2014 Jun 15;189(12):1503-8.
https://www.atsjournals.org/doi/full/10.1164/rccm.201402-0207OC#.V9weczUpW9Y
http://www.ncbi.nlm.nih.gov/pubmed/24779680?tool=bestpractice.com
Azithromycin increases the risk of colonization with macrolide-resistant organisms and should not be prescribed for patients with hearing impairment, resting tachycardia, or apparent risk of QTc prolongation.[148]Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011 Aug 25;365(8):689-98.
https://www.nejm.org/doi/full/10.1056/NEJMoa1104623
http://www.ncbi.nlm.nih.gov/pubmed/21864166?tool=bestpractice.com
Azithromycin should be considered preferentially, but not only, in former smokers with persistent exacerbations despite appropriate therapy.[1]Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2025 report. 2025 [internet publication].
https://goldcopd.org/2025-gold-report
UK guidelines advise that prophylactic azithromycin could be considered for patients who have more than three acute exacerbations requiring corticosteroid therapy and at least one exacerbation requiring hospitalization per year.[149]Smith D, Du Rand I, Addy CL, et al. British Thoracic Society guideline for the use of long-term macrolides in adults with respiratory disease. Thorax. 2020 May;75(5):370-404.
https://thorax.bmj.com/content/75/5/370
http://www.ncbi.nlm.nih.gov/pubmed/32303621?tool=bestpractice.com
Before starting prophylactic antibiotics, baseline ECG and liver function tests should be performed, a sputum sample obtained for culture and sensitivity (including tuberculosis testing), the patient's sputum clearance technique should be optimized, and bronchiectasis should be excluded with a CT scan.[2]National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management. Jul 2019 [internet publication].
https://www.nice.org.uk/guidance/ng115
[149]Smith D, Du Rand I, Addy CL, et al. British Thoracic Society guideline for the use of long-term macrolides in adults with respiratory disease. Thorax. 2020 May;75(5):370-404.
https://thorax.bmj.com/content/75/5/370
http://www.ncbi.nlm.nih.gov/pubmed/32303621?tool=bestpractice.com
ECG and liver tests should be repeated after 1 month of treatment. A head-to-head comparison of fluoroquinolones, tetracyclines, and macrolides given for 12-13 weeks to people with COPD did not identify a difference in efficacy or safety between antibiotic classes, but the sample sizes of included studies were small and the studies were of short duration; further research is required in this area.[150]Threapleton CJ, Janjua S, Fortescue R, et al. Head-to-head oral prophylactic antibiotic therapy for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2019 May 24;(5):CD013024.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD013024.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/31125127?tool=bestpractice.com
Prophylactic antibiotic therapy should be reviewed at 6 and 12 months to determine whether there is a benefit in terms of exacerbation rates.[149]Smith D, Du Rand I, Addy CL, et al. British Thoracic Society guideline for the use of long-term macrolides in adults with respiratory disease. Thorax. 2020 May;75(5):370-404.
https://thorax.bmj.com/content/75/5/370
http://www.ncbi.nlm.nih.gov/pubmed/32303621?tool=bestpractice.com
If antibiotic therapy is not effective it should be stopped.
Biologic therapies
Dupilumab, an IL-4 receptor antagonist, may be considered for patients with moderate to severe COPD taking LABA/LAMA/ICS with a history of exacerbations, chronic bronchitis, and blood eosinophils ≥300 cells/microliter.[1]Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2025 report. 2025 [internet publication].
https://goldcopd.org/2025-gold-report
[151]Bhatt SP, Rabe KF, Hanania NA, et al. Dupilumab for COPD with type 2 inflammation indicated by eosinophil counts. N Engl J Med. 2023 Jul 20;389(3):205-14.
https://www.doi.org/10.1056/NEJMoa2303951
http://www.ncbi.nlm.nih.gov/pubmed/37272521?tool=bestpractice.com
[152]Bhatt SP, Rabe KF, Hanania NA, et al. Dupilumab for COPD with blood eosinophil evidence of type 2 inflammation. N Engl J Med. 2024 Jun 27;390(24):2274-83.
http://www.ncbi.nlm.nih.gov/pubmed/38767614?tool=bestpractice.com
Dupilumab should not be used for relief of acute bronchospasm. Dupilumab is approved in the US and Europe for add-on maintenance treatment in adults with uncontrolled COPD characterized by elevated blood eosinophils.
Methylxanthines
Theophylline (a methylxanthine agent) is a bronchodilator that acts by increasing cAMP and subsequent respiratory smooth muscle relaxation. It is not commonly used because of limited potency, narrow therapeutic window, high risk profile, and frequent drug-drug interactions. Theophylline has modest effects on lung function in moderate to severe COPD.[153]Ram FSF, Jones P, Jardim J, et al. Oral theophylline for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2002;(4):CD003902.
http://www.ncbi.nlm.nih.gov/pubmed/12519617?tool=bestpractice.com
A large randomized controlled trial found no effect of oral theophylline alone or with prednisone on exacerbations of severe COPD.[154]Jenkins CR, Wen FQ, Martin A, et al; TASCS study investigators. The effect of low-dose corticosteroids and theophylline on the risk of acute exacerbations of COPD: the TASCS randomised controlled trial. Eur Respir J. 2021 Jun 10;57(6):2003338.
https://erj.ersjournals.com/content/57/6/2003338
http://www.ncbi.nlm.nih.gov/pubmed/33334939?tool=bestpractice.com
Experts may prescribe theophylline after a patient has exhausted all options for inhaled therapies. Theophylline is not recommended unless other long-term bronchodilators are unavailable or unaffordable.[1]Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2025 report. 2025 [internet publication].
https://goldcopd.org/2025-gold-report
Patient education and self-management
All patients should be well educated about the disease course and symptoms of exacerbation or decompensation. Their expectation of the disease, treatment, and prognosis should be realistic. It is important to remember that no medication has been shown to modify the long-term decline in lung function, and the primary goal of pharmacotherapy is to control symptoms and prevent complications.
One Cochrane review found that self-management interventions that include an action plan for acute exacerbations of COPD are associated with improvements in health-related quality of life and fewer admissions to the hospital for respiratory problems. An exploratory analysis found a small, but significantly higher, respiratory-related mortality rate for self-management compared to usual care, although no excess risk of all-cause mortality was seen.[155]Lenferink A, Brusse-Keizer M, van der Valk PD, et al. Self-management interventions including action plans for exacerbations versus usual care in patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017 Aug 4;(8):CD011682.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD011682.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/28777450?tool=bestpractice.com
A randomized controlled trial showed that a 3-month program of self-management started in patients with COPD exacerbations recently discharged from the hospital led to increases in COPD-related hospitalizations and emergency department visits over 6 months.[156]Aboumatar H, Naqibuddin M, Chung S, et al. Effect of a hospital-initiated program combining transitional care and long-term self-management support on outcomes of patients hospitalized with chronic obstructive pulmonary disease: a randomized clinical trial. JAMA. 2019 Oct 8;322(14):1371-80.
https://jamanetwork.com/journals/jama/fullarticle/2752467
http://www.ncbi.nlm.nih.gov/pubmed/31593271?tool=bestpractice.com
Self-management plans should include personalized advice on: breathlessness and stress management techniques; energy conservation; avoiding aggravating factors; how to monitor symptoms; how to manage worsening symptoms; and contact information to use in the event of an exacerbation.[1]Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2025 report. 2025 [internet publication].
https://goldcopd.org/2025-gold-report
Helping patients to self-manage should ideally address psychosocial concerns and patients' personal beliefs about COPD and its management. Many patients report losses and limitations on their lifestyle and social interaction after a diagnosis of COPD. It is estimated that patients with COPD are 1.9 times more likely to die from suicide than those without COPD, and symptoms of anxiety, depression, and frustration are common.[157]Sampaio MS, Vieira WA, Bernardino ÍM, et al. Chronic obstructive pulmonary disease as a risk factor for suicide: a systematic review and meta-analysis. Respir Med. 2019 May;151:11-18.
https://www.resmedjournal.com/article/S0954-6111(19)30093-9/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/31047105?tool=bestpractice.com
[158]Russell S, Ogunbayo OJ, Newham JJ, et al. Qualitative systematic review of barriers and facilitators to self-management of chronic obstructive pulmonary disease: views of patients and healthcare professionals. NPJ Prim Care Respir Med. 2018 Jan 17;28(1):2.
https://www.nature.com/articles/s41533-017-0069-z
http://www.ncbi.nlm.nih.gov/pubmed/29343739?tool=bestpractice.com
Studies have found a beneficial effect of cognitive behavioral therapy (CBT) on outcomes including symptoms of depression and anxiety, quality of life, and frequency of emergency department visits.[159]Pollok J, van Agteren JE, Esterman AJ, et al. Psychological therapies for the treatment of depression in chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2019 Mar 6;(3):CD012347.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD012347.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/30838649?tool=bestpractice.com
[160]Ma RC, Yin YY, Wang YQ, et al. Effectiveness of cognitive behavioural therapy for chronic obstructive pulmonary disease patients: a systematic review and meta-analysis. Complement Ther Clin Pract. 2020 Feb;38:101071.
http://www.ncbi.nlm.nih.gov/pubmed/31743870?tool=bestpractice.com
[161]Williams MT, Johnston KN, Paquet C. Cognitive behavioral therapy for people with chronic obstructive pulmonary disease: rapid review. Int J Chron Obstruct Pulmon Dis. 2020 Apr 23;15:903-19.
https://www.dovepress.com/cognitive-behavioral-therapy-for-people-with-chronic-obstructive-pulmo-peer-reviewed-fulltext-article-COPD
http://www.ncbi.nlm.nih.gov/pubmed/32425516?tool=bestpractice.com
Further research is warranted into the effects of high-resource-intensive versus low-resource-intensive CBT.[161]Williams MT, Johnston KN, Paquet C. Cognitive behavioral therapy for people with chronic obstructive pulmonary disease: rapid review. Int J Chron Obstruct Pulmon Dis. 2020 Apr 23;15:903-19.
https://www.dovepress.com/cognitive-behavioral-therapy-for-people-with-chronic-obstructive-pulmo-peer-reviewed-fulltext-article-COPD
http://www.ncbi.nlm.nih.gov/pubmed/32425516?tool=bestpractice.com
One randomized controlled trial found that a telephone health coaching intervention to promote behavior change in patients with mild COPD in primary care led to improvements in self-management activities, but did not improve health-related quality of life.[162]Jolly K, Sidhu MS, Hewitt CA, et al. Self management of patients with mild COPD in primary care: randomised controlled trial. BMJ. 2018 Jun 13;361:k2241.
https://www.bmj.com/content/361/bmj.k2241.long
http://www.ncbi.nlm.nih.gov/pubmed/29899047?tool=bestpractice.com
A meta-analysis found that health coaching that included goal setting, motivational interviewing, and COPD-related health education significantly improved health-related quality of life and reduced hospital admissions for an exacerbation of COPD, but did not decrease all-cause hospital admissions.[163]Long H, Howells K, Peters S, et al. Does health coaching improve health-related quality of life and reduce hospital admissions in people with chronic obstructive pulmonary disease? A systematic review and meta-analysis. Br J Health Psychol. 2019 Sep;24(3):515-46.
https://bpspsychub.onlinelibrary.wiley.com/doi/10.1111/bjhp.12366
http://www.ncbi.nlm.nih.gov/pubmed/31033121?tool=bestpractice.com
Patients who use inhaled therapies should receive training on inhaler device technique. The majority of patients make at least one error in using their inhaler, and incorrect inhaler use is associated with worse disease control.[164]Cho-Reyes S, Celli BR, Dembek C, et al. Inhalation technique errors with metered-dose inhalers among patients with obstructive lung diseases: a systematic review and meta-analysis of US Studies. Chronic Obstr Pulm Dis. 2019 Jul 24;6(3):267-80.
https://journal.copdfoundation.org/jcopdf/id/1241/Inhalation-Technique-Errors-with-Metered-Dose-Inhalers-Among-Patients-with-Obstructive-Lung-Diseases-A-Systematic-Review-and-Meta-Analysis-of-US-Studies
http://www.ncbi.nlm.nih.gov/pubmed/31342732?tool=bestpractice.com
[165]Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011 Jun;105(6):930-8.
https://www.resmedjournal.com/article/S0954-6111(11)00009-6/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/21367593?tool=bestpractice.com
Poor technique is more likely when patients are using multiple devices or have never received inhaler technique training.[166]Rootmensen GN, van Keimpema AR, Jansen HM, et al. Predictors of incorrect inhalation technique in patients with asthma or COPD: a study using a validated videotaped scoring method. J Aerosol Med Pulm Drug Deliv. 2010 Oct;23(5):323-8.
http://www.ncbi.nlm.nih.gov/pubmed/20804428?tool=bestpractice.com
Demonstration of inhaler use by a clinician, device selection, and reviewing technique at subsequent appointments can improve inhaler technique.[167]Price D, Keininger DL, Viswanad B, et al. Factors associated with appropriate inhaler use in patients with COPD - lessons from the REAL survey. Int J Chron Obstruct Pulmon Dis. 2018 Feb 26;13:695-702. [Erratum in: Int J Chron Obstruct Pulmon Dis. 2018 Jul 25;13:2253-4.]
https://www.dovepress.com/factors-associated-with-appropriate-inhaler-use-in-patients-with-copd--peer-reviewed-fulltext-article-COPD
http://www.ncbi.nlm.nih.gov/pubmed/29520137?tool=bestpractice.com
Demonstration using a placebo device may be most effective for teaching inhaler technique to adults ages ≥65 years.[168]Maricoto T, Monteiro L, Gama JMR, et al. Inhaler technique education and exacerbation risk in older adults with asthma or chronic obstructive pulmonary disease: a meta-analysis. J Am Geriatr Soc. 2019 Jan;67(1):57-66.
http://www.ncbi.nlm.nih.gov/pubmed/30291745?tool=bestpractice.com
Patients should be asked to bring their inhalers to clinic to facilitate a review of inhaler use.[1]Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2025 report. 2025 [internet publication].
https://goldcopd.org/2025-gold-report
Pharmacist-led interventions and lay health coaching can improve inhaler technique and adherence in patients with COPD.[169]Jia X, Zhou S, Luo D, et al. Effect of pharmacist-led interventions on medication adherence and inhalation technique in adult patients with asthma or COPD: a systematic review and meta-analysis. J Clin Pharm Ther. 2020 Oct;45(5):904-17.
https://onlinelibrary.wiley.com/doi/10.1111/jcpt.13126
http://www.ncbi.nlm.nih.gov/pubmed/32107837?tool=bestpractice.com
[170]Willard-Grace R, Chirinos C, Wolf J, et al. Lay health coaching to increase appropriate inhaler use in COPD: a randomized controlled trial. Ann Fam Med. 2020 Jan;18(1):5-14.
https://www.annfammed.org/content/18/1/5
http://www.ncbi.nlm.nih.gov/pubmed/31937527?tool=bestpractice.com
Inhaler device attributes such as rapid onset of symptom relief and small size have been recorded in patient preference studies.[171]Navaie M, Dembek C, Cho-Reyes S, et al. Inhaler device feature preferences among patients with obstructive lung diseases: a systematic review and meta-analysis. Medicine (Baltimore). 2020 Jun 19;99(25):e20718.
https://journals.lww.com/md-journal/fulltext/2020/06190/inhaler_device_feature_preferences_among_patients.56.aspx
http://www.ncbi.nlm.nih.gov/pubmed/32569208?tool=bestpractice.com
[172]Tervonen T, Hawken N, Hanania NA, et al. Maintenance inhaler therapy preferences of patients with asthma or chronic obstructive pulmonary disease: a discrete choice experiment. Thorax. 2020 Sep;75(9):735-43.
https://thorax.bmj.com/content/75/9/735
http://www.ncbi.nlm.nih.gov/pubmed/32631932?tool=bestpractice.com
Metered dose inhalerA principal pharmacist shows a patient how to use a metered dose inhaler and discusses ways of improving inhaler technique.
Metered dose inhaler plus spacerA principal pharmacist shows a patient how to use a metered dose inhaler plus a spacer and discusses ways of improving inhaler technique.
Dry powder inhalersA principal pharmacist shows a patient how to use dry powder devices and discusses ways of improving inhaler technique.
Soft mist inhalerA principal pharmacist shows a patient how to use a soft mist inhaler and discusses ways of improving inhaler technique.
Physical activity is recommended for all patients with COPD.[1]Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2025 report. 2025 [internet publication].
https://goldcopd.org/2025-gold-report
One systematic review and meta-analysis of randomized controlled trials found that exercise training on its own can improve physical activity in COPD, and greater improvements can be made with the addition of physical activity counseling.[173]Lahham A, McDonald CF, Holland AE. Exercise training alone or with the addition of activity counseling improves physical activity levels in COPD: a systematic review and meta-analysis of randomized controlled trials. Int J Chron Obstruct Pulmon Dis. 2016 Dec 8;11:3121-36.
https://www.dovepress.com/exercise-training-alone-or-with-the-addition-of-activity-counseling-im-peer-reviewed-fulltext-article-COPD
http://www.ncbi.nlm.nih.gov/pubmed/27994451?tool=bestpractice.com
Another systematic review and meta-analysis found that a combination of aerobic exercise and strength training was more effective than strength training or endurance training alone in increasing the 6-minute walking distance.[174]Vooijs M, Siemonsma PC, Heus I, et al. Therapeutic validity and effectiveness of supervised physical exercise training on exercise capacity in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Clin Rehabil. 2016 Nov;30(11):1037-48.
http://www.ncbi.nlm.nih.gov/pubmed/26451006?tool=bestpractice.com
Other studies have demonstrated improvements in peak oxygen uptake, perceived fatigue, and health-related quality of life following adherence to supervised and unsupervised exercise programs.[175]Ward TJC, Plumptre CD, Dolmage TE, et al. Change in VO2peak in response to aerobic exercise training and the relationship with exercise prescription in people with COPD: a systematic review and meta-analysis. Chest. 2020 Jul;158(1):131-44.
http://www.ncbi.nlm.nih.gov/pubmed/32173489?tool=bestpractice.com
[176]Paneroni M, Vitacca M, Venturelli M, et al. The impact of exercise training on fatigue in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Pulmonology. 2020 Sep - Oct;26(5):304-13.
http://www.ncbi.nlm.nih.gov/pubmed/32184070?tool=bestpractice.com
[177]Taylor D, Jenkins AR, Parrott K, et al. Efficacy of unsupervised exercise in adults with obstructive lung disease: a systematic review and meta-analysis. Thorax. 2021 Jun;76(6):591-600.
http://www.ncbi.nlm.nih.gov/pubmed/33685962?tool=bestpractice.com
One Cochrane review found limited evidence for improvement in physical activity with physical activity counseling, exercise training, and pharmacologic management of COPD. The authors commented that assessment of quality had been limited by lack of methodologic detail and the diverse range of interventions had primarily been assessed in single studies.[178]Burge AT, Cox NS, Abramson MJ, et al. Interventions for promoting physical activity in people with chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2020 Apr 16;(4):CD012626.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD012626.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/32297320?tool=bestpractice.com
[  ]
Which interventions lead to increased regular physical activity for people with chronic obstructive pulmonary disease (COPD)?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.3155/fullShow me the answer The optimal timing, components, duration, and models for improving physical activity remain unclear. Meta-analyses suggest that yoga, Qigong, and other home-based breathing exercises can improve exercise capacity and pulmonary function in patients with COPD.[179]Cramer H, Haller H, Klose P, et al. The risks and benefits of yoga for patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Clin Rehabil. 2019 Dec;33(12):1847-62.
http://www.ncbi.nlm.nih.gov/pubmed/31353959?tool=bestpractice.com
[180]Tong H, Liu Y, Zhu Y, et al. The therapeutic effects of qigong in patients with chronic obstructive pulmonary disease in the stable stage: a meta-analysis. BMC Complement Altern Med. 2019 Sep 4;19(1):239.
https://bmccomplementmedtherapies.biomedcentral.com/articles/10.1186/s12906-019-2639-9
http://www.ncbi.nlm.nih.gov/pubmed/31484521?tool=bestpractice.com
[181]Lu Y, Li P, Li N, et al. Effects of home-based breathing exercises in subjects with COPD. Respir Care. 2020 Mar;65(3):377-87.
https://rc.rcjournal.com/content/65/3/377
http://www.ncbi.nlm.nih.gov/pubmed/31719191?tool=bestpractice.com
Tai Chi has been shown to improve exercise capacity compared with usual care.[182]Liu X, Fu C, Hu W, et al. The effect of Tai Chi on the pulmonary rehabilitation of chronic obstructive pulmonary disease: a systematic review and meta-analysis. Ann Palliat Med. 2021 Apr;10(4):3763-82.
https://apm.amegroups.org/article/view/67333/html
http://www.ncbi.nlm.nih.gov/pubmed/33894710?tool=bestpractice.com
]
Which interventions lead to increased regular physical activity for people with chronic obstructive pulmonary disease (COPD)?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.3155/fullShow me the answer The optimal timing, components, duration, and models for improving physical activity remain unclear. Meta-analyses suggest that yoga, Qigong, and other home-based breathing exercises can improve exercise capacity and pulmonary function in patients with COPD.[179]Cramer H, Haller H, Klose P, et al. The risks and benefits of yoga for patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Clin Rehabil. 2019 Dec;33(12):1847-62.
http://www.ncbi.nlm.nih.gov/pubmed/31353959?tool=bestpractice.com
[180]Tong H, Liu Y, Zhu Y, et al. The therapeutic effects of qigong in patients with chronic obstructive pulmonary disease in the stable stage: a meta-analysis. BMC Complement Altern Med. 2019 Sep 4;19(1):239.
https://bmccomplementmedtherapies.biomedcentral.com/articles/10.1186/s12906-019-2639-9
http://www.ncbi.nlm.nih.gov/pubmed/31484521?tool=bestpractice.com
[181]Lu Y, Li P, Li N, et al. Effects of home-based breathing exercises in subjects with COPD. Respir Care. 2020 Mar;65(3):377-87.
https://rc.rcjournal.com/content/65/3/377
http://www.ncbi.nlm.nih.gov/pubmed/31719191?tool=bestpractice.com
Tai Chi has been shown to improve exercise capacity compared with usual care.[182]Liu X, Fu C, Hu W, et al. The effect of Tai Chi on the pulmonary rehabilitation of chronic obstructive pulmonary disease: a systematic review and meta-analysis. Ann Palliat Med. 2021 Apr;10(4):3763-82.
https://apm.amegroups.org/article/view/67333/html
http://www.ncbi.nlm.nih.gov/pubmed/33894710?tool=bestpractice.com
Dietary advice and oral supplements have been found to improve body weight, quality of life, respiratory muscle strength, and 6-minute walk distance.[183]Collins PF, Elia M, Stratton RJ. Nutritional support and functional capacity in chronic obstructive pulmonary disease: a systematic review and meta-analysis. Respirology. 2013 May;18(4):616-29.
https://onlinelibrary.wiley.com/doi/10.1111/resp.12070
http://www.ncbi.nlm.nih.gov/pubmed/23432923?tool=bestpractice.com
[184]Ferreira IM, Brooks D, White J, et al. Nutritional supplementation for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012 Dec 12;(12):CD000998.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD000998.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/23235577?tool=bestpractice.com
However, nutritional support has not been consistently found to improve lung function.[184]Ferreira IM, Brooks D, White J, et al. Nutritional supplementation for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012 Dec 12;(12):CD000998.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD000998.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/23235577?tool=bestpractice.com
Smoking cessation
Smoking cessation should be encouraged in all patients, in addition to guidance on avoiding exposure to occupational or environmental tobacco smoke and other irritants.[1]Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2025 report. 2025 [internet publication].
https://goldcopd.org/2025-gold-report
[2]National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management. Jul 2019 [internet publication].
https://www.nice.org.uk/guidance/ng115
Smoking cessation significantly reduces the rate of progression of COPD and risk of malignancies. It also reduces the risk of coronary and cerebrovascular diseases. Among different therapeutic modalities in COPD, the only two factors that improve survival are smoking cessation and oxygen supplementation.
Usual smoking-cessation programs include counseling, group meetings, and drug therapy.[185]Gonzales D, Rennard SI, Nides M, et al; Varenicline Phase 3 Study Group. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006 Jul 5;296(1):47-55.
https://jamanetwork.com/journals/jama/fullarticle/211000
http://www.ncbi.nlm.nih.gov/pubmed/16820546?tool=bestpractice.com
Some patients may need frequent referrals to achieve success. Smoking cessation that includes pharmacotherapy and intensive counseling has a higher success rate and is cost effective in COPD, with low costs per quality-adjusted life year.[186]Hoogendoorn M, Feenstra TL, Hoogenveen RT, et al. Long-term effectiveness and cost-effectiveness of smoking cessation interventions in patients with COPD. Thorax. 2010 Aug;65(8):711-8.
http://www.ncbi.nlm.nih.gov/pubmed/20685746?tool=bestpractice.com
[187]Warnier MJ, van Riet EE, Rutten FH, et al. Smoking cessation strategies in patients with COPD. Eur Respir J. 2013 Mar;41(3):727-34.
http://www.ncbi.nlm.nih.gov/pubmed/22936706?tool=bestpractice.com
[188]van Eerd EA, van der Meer RM, van Schayck OC, et al. Smoking cessation for people with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2016 Aug 20;(8):CD010744.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD010744.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/27545342?tool=bestpractice.com
The use of electronic nicotine delivery systems (ENDS), including e-cigarette and vaping products, as tobacco cessation aids is controversial. Evidence from observational studies does not currently support the use of ENDS as an aid to smoking cessation in people with COPD.[189]Bowler RP, Hansel NN, Jacobson S, et al; for COPDGene and SPIROMICS Investigators. Electronic cigarette use in US adults at risk for or with COPD: analysis from two observational cohorts. J Gen Intern Med. 2017 Dec;32(12):1315-22.
https://link.springer.com/article/10.1007/s11606-017-4150-7
http://www.ncbi.nlm.nih.gov/pubmed/28884423?tool=bestpractice.com
See Smoking cessation (Management approach).
Mucolytics
Patients with the chronic bronchitis phenotype of COPD often produce thick sputum on a frequent basis. Mucolytic agents (erdosteine, carbocysteine, and acetylcysteine) are not associated with an increase in adverse effects and may be beneficial during exacerbations of COPD.
[  ]
For people with chronic bronchitis or chronic obstructive pulmonary disease, how do mucolytic agents compare with placebo?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2591/fullShow me the answer They result in a small reduction in the frequency of acute exacerbations and in days of disability per month, but do not improve lung function or quality of life.[190]Poole P, Sathananthan K, Fortescue R. Mucolytic agents versus placebo for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2019 May 20;(5):CD001287.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD001287.pub6/full
http://www.ncbi.nlm.nih.gov/pubmed/31107966?tool=bestpractice.com
[191]Rogliani P, Matera MG, Page C, et al. Efficacy and safety profile of mucolytic/antioxidant agents in chronic obstructive pulmonary disease: a comparative analysis across erdosteine, carbocysteine, and N-acetylcysteine. Respir Res. 2019 May 27;20(1):104.
https://respiratory-research.biomedcentral.com/articles/10.1186/s12931-019-1078-y
http://www.ncbi.nlm.nih.gov/pubmed/31133026?tool=bestpractice.com
[192]Wei J, Pang CS, Han J, et al. Effect of orally administered N-acetylcysteine on chronic bronchitis: a meta-analysis. Adv Ther. 2019 Dec;36(12):3356-67.
http://www.ncbi.nlm.nih.gov/pubmed/31598901?tool=bestpractice.com
Erdosteine and carbocysteine are not available in the US. Treatment with mucolytic agents such as carbocysteine and acetylcysteine may reduce exacerbations and modestly improve health status in patients not receiving ICS.[1]Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2025 report. 2025 [internet publication].
https://goldcopd.org/2025-gold-report
However, erdosteine may have a significant effect on mild exacerbations whether or not the patient is taking ICS.[1]Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2025 report. 2025 [internet publication].
https://goldcopd.org/2025-gold-report
]
For people with chronic bronchitis or chronic obstructive pulmonary disease, how do mucolytic agents compare with placebo?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2591/fullShow me the answer They result in a small reduction in the frequency of acute exacerbations and in days of disability per month, but do not improve lung function or quality of life.[190]Poole P, Sathananthan K, Fortescue R. Mucolytic agents versus placebo for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2019 May 20;(5):CD001287.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD001287.pub6/full
http://www.ncbi.nlm.nih.gov/pubmed/31107966?tool=bestpractice.com
[191]Rogliani P, Matera MG, Page C, et al. Efficacy and safety profile of mucolytic/antioxidant agents in chronic obstructive pulmonary disease: a comparative analysis across erdosteine, carbocysteine, and N-acetylcysteine. Respir Res. 2019 May 27;20(1):104.
https://respiratory-research.biomedcentral.com/articles/10.1186/s12931-019-1078-y
http://www.ncbi.nlm.nih.gov/pubmed/31133026?tool=bestpractice.com
[192]Wei J, Pang CS, Han J, et al. Effect of orally administered N-acetylcysteine on chronic bronchitis: a meta-analysis. Adv Ther. 2019 Dec;36(12):3356-67.
http://www.ncbi.nlm.nih.gov/pubmed/31598901?tool=bestpractice.com
Erdosteine and carbocysteine are not available in the US. Treatment with mucolytic agents such as carbocysteine and acetylcysteine may reduce exacerbations and modestly improve health status in patients not receiving ICS.[1]Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2025 report. 2025 [internet publication].
https://goldcopd.org/2025-gold-report
However, erdosteine may have a significant effect on mild exacerbations whether or not the patient is taking ICS.[1]Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2025 report. 2025 [internet publication].
https://goldcopd.org/2025-gold-report
Pulmonary rehabilitation
Pulmonary rehabilitation comprises aerobic exercise, strength training, and education. It should be initiated for patients who remain symptomatic despite bronchodilator therapy and is recommended to start early in the course of the disease, when they start feeling shortness of breath with regular activity and walking on a level surface.[1]Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2025 report. 2025 [internet publication].
https://goldcopd.org/2025-gold-report
[193]Rochester CL, Alison JA, Carlin B, et al. Pulmonary rehabilitation for adults with chronic respiratory disease: an official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2023 Aug 15;208(4):e7-26.
https://www.atsjournals.org/doi/10.1164/rccm.202306-1066ST
http://www.ncbi.nlm.nih.gov/pubmed/37581410?tool=bestpractice.com
[194]Man W, Chaplin E, Daynes E, et al. British Thoracic Society Clinical Statement on pulmonary rehabilitation. Thorax. 2023 Oct;78(suppl 5):s2-15.
https://thorax.bmj.com/content/78/Suppl_5/s2
http://www.ncbi.nlm.nih.gov/pubmed/37770084?tool=bestpractice.com
GOLD guidelines recommend pulmonary rehabilitation for patients with high symptom burden and risk of exacerbation (i.e., groups B and E).[1]Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2025 report. 2025 [internet publication].
https://goldcopd.org/2025-gold-report
Pulmonary rehabilitation relieves dyspnea and fatigue, improves emotional function, and enhances a sense of control to a moderately large and clinically significant extent.[195]McCarthy B, Casey D, Devane D, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015 Feb 23;(2):CD003793.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD003793.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/25705944?tool=bestpractice.com
Extensive pulmonary rehabilitation following hospital admission with an acute exacerbation of COPD decreases the risk of readmission, improves health-related quality of life, and reduces mortality.
[  ]
What are the effects of pulmonary rehabilitation after exacerbation in people with chronic obstructive pulmonary disease?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1650/fullShow me the answer There is evidence to support starting pulmonary rehabilitation within 1 month of an acute exacerbation.[196]Marciniuk DD, Brooks D, Butcher S, et al. Canadian Thoracic Society COPD Committee Expert Working Group. Optimizing pulmonary rehabilitation in chronic obstructive pulmonary disease - practical issues: a Canadian Thoracic Society Clinical Practice Guideline. Can Respir J. 2010 Jul-Aug;17(4):159-68.
http://www.ncbi.nlm.nih.gov/pubmed/20808973?tool=bestpractice.com
[197]Puhan MA, Gimeno-Santos E, Cates CJ, et al. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2016 Dec 8;(12):CD005305.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD005305.pub4/full
http://www.ncbi.nlm.nih.gov/pubmed/27930803?tool=bestpractice.com
]
What are the effects of pulmonary rehabilitation after exacerbation in people with chronic obstructive pulmonary disease?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1650/fullShow me the answer There is evidence to support starting pulmonary rehabilitation within 1 month of an acute exacerbation.[196]Marciniuk DD, Brooks D, Butcher S, et al. Canadian Thoracic Society COPD Committee Expert Working Group. Optimizing pulmonary rehabilitation in chronic obstructive pulmonary disease - practical issues: a Canadian Thoracic Society Clinical Practice Guideline. Can Respir J. 2010 Jul-Aug;17(4):159-68.
http://www.ncbi.nlm.nih.gov/pubmed/20808973?tool=bestpractice.com
[197]Puhan MA, Gimeno-Santos E, Cates CJ, et al. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2016 Dec 8;(12):CD005305.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD005305.pub4/full
http://www.ncbi.nlm.nih.gov/pubmed/27930803?tool=bestpractice.com
A large US cohort study found that initiation of pulmonary rehabilitation within 90 days of hospital discharge was significantly associated with lower mortality risk at 1 year and fewer rehospitalizations at 1 year.[198]Lindenauer PK, Stefan MS, Pekow PS, et al. Association between initiation of pulmonary rehabilitation after hospitalization for COPD and 1-year survival among medicare beneficiaries. JAMA. 2020 May 12;323(18):1813-23.
https://jamanetwork.com/journals/jama/fullarticle/2765730
http://www.ncbi.nlm.nih.gov/pubmed/32396181?tool=bestpractice.com
[199]Stefan MS, Pekow PS, Priya A, et al. Association between initiation of pulmonary rehabilitation and rehospitalizations in patients hospitalized with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2021 Nov 1;204(9):1015-23.
https://www.atsjournals.org/doi/10.1164/rccm.202012-4389OC
http://www.ncbi.nlm.nih.gov/pubmed/34283694?tool=bestpractice.com
Less than 2% of the patient cohort initiated rehabilitation within this timeframe, highlighting the need to develop more effective strategies to encourage patient participation.[198]Lindenauer PK, Stefan MS, Pekow PS, et al. Association between initiation of pulmonary rehabilitation after hospitalization for COPD and 1-year survival among medicare beneficiaries. JAMA. 2020 May 12;323(18):1813-23.
https://jamanetwork.com/journals/jama/fullarticle/2765730
http://www.ncbi.nlm.nih.gov/pubmed/32396181?tool=bestpractice.com
However, starting pulmonary rehabilitation before hospital discharge could be associated with a higher 12-month mortality, so is not recommended.[200]Greening NJ, Williams JE, Hussain SF, et al. An early rehabilitation intervention to enhance recovery during hospital admission for an exacerbation of chronic respiratory disease: randomised controlled trial. BMJ. 2014 Jul 8;349:g4315.
https://www.bmj.com/content/349/bmj.g4315
http://www.ncbi.nlm.nih.gov/pubmed/25004917?tool=bestpractice.com
Pulmonary rehabilitation also decreases the depression and anxiety related to COPD, and reduces hospitalization.[201]Casaburi R, ZuWallack R. Pulmonary rehabilitation for management of chronic obstructive pulmonary disease. N Engl J Med. 2009 Mar 26;360(13):1329-35.
http://www.ncbi.nlm.nih.gov/pubmed/19321869?tool=bestpractice.com
The benefit of pulmonary rehabilitation appears to subside after termination of the course unless patients follow a home exercise schedule.[202]Guell R, Casan P, Belda J, et al. Long-term effects of outpatient rehabilitation of COPD: a randomized trial. Chest. 2000 Apr;117(4):976-83.
http://www.ncbi.nlm.nih.gov/pubmed/10767227?tool=bestpractice.com
Maintenance pulmonary rehabilitation, defined as ongoing supervised exercise at a lower frequency than the original rehabilitation program, may have a role in preserving the benefits of pulmonary rehabilitation over time. Findings from one Cochrane review indicate that supervised maintenance programs may improve health-related quality of life and exercise capacity at 6-12 months compared with usual care.[203]Malaguti C, Dal Corso S, Janjua S, et al. Supervised maintenance programmes following pulmonary rehabilitation compared to usual care for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2021 Aug 17;(8):CD013569.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD013569.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/34404111?tool=bestpractice.com
[  ]
For people with chronic obstructive pulmonary disease (COPD), what are the effects of a supervised maintenance program after pulmonary rehabilitation?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.4228/fullShow me the answer
]
For people with chronic obstructive pulmonary disease (COPD), what are the effects of a supervised maintenance program after pulmonary rehabilitation?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.4228/fullShow me the answer
Benefits of home- or community-based pulmonary rehabilitation on respiratory symptoms and quality of life in patients with COPD can match those of the hospital-based rehabilitation programs.[204]Maltais F, Bourbeau J, Shapiro S, et al. Effects of home-based pulmonary rehabilitation in patients with chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2008 Dec 16;149(12):869-78.
http://www.ncbi.nlm.nih.gov/pubmed/19075206?tool=bestpractice.com
[205]Neves LF, Reis MH, Gonçalves TR. Home or community-based pulmonary rehabilitation for individuals with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Cad Saude Publica. 2016 Jun 20;32(6):S0102-311X2016000602001.
http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0102-311X2016000602001&lng=en&nrm=iso&tlng=en
http://www.ncbi.nlm.nih.gov/pubmed/27333130?tool=bestpractice.com
[206]Holland AE, Mahal A, Hill CJ, et al. Home-based rehabilitation for COPD using minimal resources: a randomised, controlled equivalence trial. Thorax. 2017 Jan;72(1):57-65.
https://thorax.bmj.com/content/72/1/57
http://www.ncbi.nlm.nih.gov/pubmed/27672116?tool=bestpractice.com
One Cochrane review concluded that both primary and maintenance telerehabilitation achieved similar outcomes to in-person rehabilitation with no safety issues. Limitations of the review include small patient numbers and heterogeneity in telerehabilitation models.[207]Cox NS, Dal Corso S, Hansen H, et al. Telerehabilitation for chronic respiratory disease. Cochrane Database Syst Rev. 2021 Jan 29;(1):CD013040.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD013040.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/33511633?tool=bestpractice.com
Oxygen therapy and ventilatory support
GOLD guidelines recommend long-term oxygen therapy in stable patients who have:[1]Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2025 report. 2025 [internet publication].
https://goldcopd.org/2025-gold-report
PaO₂ ≤7.3 kPa (55 mmHg) or SaO₂ ≤88%, with or without hypercapnia confirmed twice over a 3-week period; or
PaO₂ between 7.3 kPa (55 mmHg) and 8.0 kPa (60 mmHg), or SaO₂ of 88%, if there is evidence of pulmonary hypertension, peripheral edema suggesting congestive cardiac failure, or polycythemia (hematocrit >55%).
Guidelines from the American Thoracic Society (ATS) recommend prescribing long-term oxygen therapy for at least 15 hours per day in adults with COPD who have severe chronic resting room air hypoxemia. The ATS defines severe hypoxemia as either:[208]Jacobs SS, Krishnan JA, Lederer DJ, et al. Home oxygen therapy for adults with chronic lung disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020 Nov 15;202(10):e121-41. [Erratum in: Am J Respir Crit Care Med. 2021 Apr 15;203(8):1045-6.]
https://www.atsjournals.org/doi/10.1164/rccm.202009-3608ST
http://www.ncbi.nlm.nih.gov/pubmed/33185464?tool=bestpractice.com
PaO₂ ≤7.3 kPa (55 mmHg) or oxygen saturation as measured by pulse oximetry (SpO₂) ≤88%; or
PaO₂ 7.5 to 7.9 kPa (56-59 mmHg) or SpO₂ of 89% plus one of the following: edema, hematocrit ≥55%, or P pulmonale on an ECG.
For patients prescribed home oxygen therapy, the ATS recommends that the patient and their caregivers should receive instruction and training on the use and maintenance of all oxygen equipment and education on oxygen safety, including smoking cessation, fire prevention, and tripping hazards.[208]Jacobs SS, Krishnan JA, Lederer DJ, et al. Home oxygen therapy for adults with chronic lung disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020 Nov 15;202(10):e121-41. [Erratum in: Am J Respir Crit Care Med. 2021 Apr 15;203(8):1045-6.]
https://www.atsjournals.org/doi/10.1164/rccm.202009-3608ST
http://www.ncbi.nlm.nih.gov/pubmed/33185464?tool=bestpractice.com
Supplemental oxygen should be titrated to achieve SaO₂ ≥90%.[1]Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2025 report. 2025 [internet publication].
https://goldcopd.org/2025-gold-report
The patient should be reassessed after 60-90 days to determine whether oxygen is still indicated and is therapeutic.[1]Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2025 report. 2025 [internet publication].
https://goldcopd.org/2025-gold-report
Among different therapeutic modalities in COPD, the only two factors that improve survival are smoking cessation and oxygen supplementation.
Oxygen therapy helps minimize pulmonary hypertension by decreasing pulmonary artery pressure, and improves exercise tolerance and quality of life. It has been shown to improve survival.[1]Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2025 report. 2025 [internet publication].
https://goldcopd.org/2025-gold-report
[66]Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011 Aug 2;155(3):179-91.
https://www.acpjournals.org/doi/10.7326/0003-4819-155-3-201108020-00008
http://www.ncbi.nlm.nih.gov/pubmed/21810710?tool=bestpractice.com
There is some evidence that oxygen can relieve breathlessness when given during exercise to mildly hypoxemic and nonhypoxemic people with COPD who do not otherwise qualify for home oxygen therapy.[209]Ekström M, Ahmadi Z, Bornefalk-Hermansson A, et al. Oxygen for breathlessness in patients with chronic obstructive pulmonary disease who do not qualify for home oxygen therapy. Cochrane Database Syst Rev. 2016 Nov 25;(11):CD006429.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD006429.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/27886372?tool=bestpractice.com
The ATS suggests prescribing ambulatory oxygen (oxygen delivered during exercise or activities of daily living) in adults with COPD who have severe exertional room air hypoxemia.[208]Jacobs SS, Krishnan JA, Lederer DJ, et al. Home oxygen therapy for adults with chronic lung disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020 Nov 15;202(10):e121-41. [Erratum in: Am J Respir Crit Care Med. 2021 Apr 15;203(8):1045-6.]
https://www.atsjournals.org/doi/10.1164/rccm.202009-3608ST
http://www.ncbi.nlm.nih.gov/pubmed/33185464?tool=bestpractice.com
However, the ATS suggests not prescribing long-term oxygen therapy in adults with COPD who have moderate chronic resting room air hypoxemia (SpO₂ of 89% to 93%).[208]Jacobs SS, Krishnan JA, Lederer DJ, et al. Home oxygen therapy for adults with chronic lung disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020 Nov 15;202(10):e121-41. [Erratum in: Am J Respir Crit Care Med. 2021 Apr 15;203(8):1045-6.]
https://www.atsjournals.org/doi/10.1164/rccm.202009-3608ST
http://www.ncbi.nlm.nih.gov/pubmed/33185464?tool=bestpractice.com
Air travel is safe for most patients receiving long-term oxygen therapy, although patients should be able to tolerate short periods without oxygen as it is likely there will be times it is unavailable.[1]Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2025 report. 2025 [internet publication].
https://goldcopd.org/2025-gold-report
[210]British Thoracic Society. Air travel. Mar 2022 [internet publication].
https://www.brit-thoracic.org.uk/quality-improvement/clinical-statements/air-travel
Patients with SaO₂ >95% at sea level and SaO₂ ≥84% after a 6-minute walk test may travel by air without further assessment.[1]Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2025 report. 2025 [internet publication].
https://goldcopd.org/2025-gold-report
[210]British Thoracic Society. Air travel. Mar 2022 [internet publication].
https://www.brit-thoracic.org.uk/quality-improvement/clinical-statements/air-travel
[211]Edvardsen A, Akerø A, Christensen CC, et al. Air travel and chronic obstructive pulmonary disease: a new algorithm for pre-flight evaluation. Thorax. 2012 Nov;67(11):964-9.
https://thorax.bmj.com/content/67/11/964
http://www.ncbi.nlm.nih.gov/pubmed/22767877?tool=bestpractice.com
Supplemental oxygen is recommended for patients with SaO₂ 92% to 95% at sea level and SaO₂ <84% after a 6-minute walk test, and for patients with SaO₂ <92% at sea level.[211]Edvardsen A, Akerø A, Christensen CC, et al. Air travel and chronic obstructive pulmonary disease: a new algorithm for pre-flight evaluation. Thorax. 2012 Nov;67(11):964-9.
https://thorax.bmj.com/content/67/11/964
http://www.ncbi.nlm.nih.gov/pubmed/22767877?tool=bestpractice.com
Hypoxia-altitude simulation testing (also known as hypoxic challenge testing) should be performed for other patients.[210]British Thoracic Society. Air travel. Mar 2022 [internet publication].
https://www.brit-thoracic.org.uk/quality-improvement/clinical-statements/air-travel
[211]Edvardsen A, Akerø A, Christensen CC, et al. Air travel and chronic obstructive pulmonary disease: a new algorithm for pre-flight evaluation. Thorax. 2012 Nov;67(11):964-9.
https://thorax.bmj.com/content/67/11/964
http://www.ncbi.nlm.nih.gov/pubmed/22767877?tool=bestpractice.com
For patients on long-term oxygen therapy, the British Thoracic Society recommends considering in-flight oxygen at 2L/minute more than the patient's usual prescription.[210]British Thoracic Society. Air travel. Mar 2022 [internet publication].
https://www.brit-thoracic.org.uk/quality-improvement/clinical-statements/air-travel
For patients who have COPD and obstructive sleep apnea, ventilatory support with continuous positive airway pressure (CPAP) can improve survival and reduce hospital admissions.[1]Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2025 report. 2025 [internet publication].
https://goldcopd.org/2025-gold-report
[73]Marin JM, Soriano JB, Carrizo SJ, et al. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010 Aug 1;182(3):325-31.
https://www.atsjournals.org/doi/10.1164/rccm.200912-1869OC
http://www.ncbi.nlm.nih.gov/pubmed/20378728?tool=bestpractice.com
Noninvasive ventilation (NIV) is occasionally used in patients with very severe but stable COPD, although the optimal timing for initiation and best selection criteria for candidates is unclear.[1]Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2025 report. 2025 [internet publication].
https://goldcopd.org/2025-gold-report
[212]Wilson ME, Dobler CC, Morrow AS, et al. Association of home noninvasive positive pressure ventilation with clinical outcomes in chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2020 Feb 4;323(5):455-65.
https://jamanetwork.com/journals/jama/fullarticle/2760390
http://www.ncbi.nlm.nih.gov/pubmed/32016309?tool=bestpractice.com
[213]Raveling T, Vonk J, Struik FM, et al. Chronic non-invasive ventilation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2021 Aug 9;(8):CD002878.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD002878.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/34368950?tool=bestpractice.com
One Cochrane review found that chronic NIV delivered via a facial mask improved survival and conferred short-term health-related quality of life benefit in stable COPD. Chronic NIV also improved duration of hospital admission-free survival in patients with persistent hypercapnia following an exacerbation.[213]Raveling T, Vonk J, Struik FM, et al. Chronic non-invasive ventilation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2021 Aug 9;(8):CD002878.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD002878.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/34368950?tool=bestpractice.com
Another study reported a significant decrease in exacerbation frequency with NIV versus control therapy, although no improvements were observed in mortality, PaO₂, PaCO₂, or pH.[214]He X, Luo L, Ma Y, et al. Efficacy of domiciliary noninvasive ventilation on clinical outcomes in posthospital chronic obstructive pulmonary disease patients: a meta-analysis of randomized controlled trials. Ann Palliat Med. 2021 May;10(5):5137-45.
http://www.ncbi.nlm.nih.gov/pubmed/33977751?tool=bestpractice.com
Guidelines from the ATS suggest the use of nocturnal NIV in addition to usual care for patients with chronic stable hypercapnic COPD.[215]Macrea M, Oczkowski S, Rochwerg B, et al. Long-term noninvasive ventilation in chronic stable hypercapnic chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice fuideline. Am J Respir Crit Care Med. 2020 Aug 15;202(4):e74-87.
https://www.atsjournals.org/doi/10.1164/rccm.202006-2382ST
http://www.ncbi.nlm.nih.gov/pubmed/32795139?tool=bestpractice.com
The European Respiratory Society and Canadian Thoracic Society have issued similar guidance.[216]Ergan B, Oczkowski S, Rochwerg B, et al. European Respiratory Society guidelines on long-term home non-invasive ventilation for management of COPD. Eur Respir J. 2019 Sep 28;54(3):1901003.
https://erj.ersjournals.com/content/54/3/1901003
http://www.ncbi.nlm.nih.gov/pubmed/31467119?tool=bestpractice.com
[217]Kaminska M, Rimmer KP, McKim DA, et al. Long-term non-invasive ventilation in patients with chronic obstructive pulmonary disease (COPD): 2021 Canadian Thoracic Society clinical practice guideline update. Can J Respir Crit Care Sleep Med. 2021 May 7;5(3):160-83.
https://www.tandfonline.com/doi/full/10.1080/24745332.2021.1911218
Surgery
Surgical interventions are the last step in the management of COPD, and include bullectomy, lung volume reduction surgery, and lung transplant.[218]van Agteren JE, Hnin K, Grosser D, et al. Bronchoscopic lung volume reduction procedures for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017 Feb 23;(2):CD012158.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD012158.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/28230230?tool=bestpractice.com
[219]van Agteren JE, Carson KV, Tiong LU, et al. Lung volume reduction surgery for diffuse emphysema. Cochrane Database Syst Rev. 2016 Oct 14;(10):CD001001.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD001001.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/27739074?tool=bestpractice.com
[  ]
How does bronchoscopic lung volume reduction compare with medical therapy in people with chronic obstructive pulmonary disease?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1680/fullShow me the answer They are used to improve lung dynamics, exercise adherence, and quality of life.[219]van Agteren JE, Carson KV, Tiong LU, et al. Lung volume reduction surgery for diffuse emphysema. Cochrane Database Syst Rev. 2016 Oct 14;(10):CD001001.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD001001.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/27739074?tool=bestpractice.com
Lung volume reduction surgery is indicated in patients with very severe airflow limitation, and especially in patients with localized upper lobe disease and lower than normal exercise capacity.[218]van Agteren JE, Hnin K, Grosser D, et al. Bronchoscopic lung volume reduction procedures for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017 Feb 23;(2):CD012158.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD012158.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/28230230?tool=bestpractice.com
[
]
How does bronchoscopic lung volume reduction compare with medical therapy in people with chronic obstructive pulmonary disease?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1680/fullShow me the answer They are used to improve lung dynamics, exercise adherence, and quality of life.[219]van Agteren JE, Carson KV, Tiong LU, et al. Lung volume reduction surgery for diffuse emphysema. Cochrane Database Syst Rev. 2016 Oct 14;(10):CD001001.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD001001.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/27739074?tool=bestpractice.com
Lung volume reduction surgery is indicated in patients with very severe airflow limitation, and especially in patients with localized upper lobe disease and lower than normal exercise capacity.[218]van Agteren JE, Hnin K, Grosser D, et al. Bronchoscopic lung volume reduction procedures for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017 Feb 23;(2):CD012158.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD012158.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/28230230?tool=bestpractice.com
[  ]
How does bronchoscopic lung volume reduction compare with medical therapy in people with chronic obstructive pulmonary disease?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1680/fullShow me the answer One meta-analysis found an increased risk of early mortality in patients who underwent lung volume reduction surgery compared to standard care; however, no significant difference was observed in overall mortality.[220]van Geffen WH, Slebos DJ, Herth FJ, et al. Surgical and endoscopic interventions that reduce lung volume for emphysema: a systemic review and meta-analysis. Lancet Respir Med. 2019 Apr;7(4):313-24.
http://www.ncbi.nlm.nih.gov/pubmed/30744937?tool=bestpractice.com
Bullectomy is an option in COPD patients with dyspnea in whom CT reveals huge bullae occupying at least 30% of the hemithorax. Severely poor functional status and severe decrease in FEV₁ (<500 mL) make these options less favorable. Endobronchial valve insertion can produce clinically meaningful improvements in appropriately selected patients with COPD.[220]van Geffen WH, Slebos DJ, Herth FJ, et al. Surgical and endoscopic interventions that reduce lung volume for emphysema: a systemic review and meta-analysis. Lancet Respir Med. 2019 Apr;7(4):313-24.
http://www.ncbi.nlm.nih.gov/pubmed/30744937?tool=bestpractice.com
[221]Klooster K, Slebos DJ, Zoumot Z, et al. Endobronchial valves for emphysema: an individual patient-level reanalysis of randomised controlled trials. BMJ Open Respir Res. 2017 Nov 2;4(1):e000214.
https://bmjopenrespres.bmj.com/content/4/1/e000214
http://www.ncbi.nlm.nih.gov/pubmed/29441206?tool=bestpractice.com
[222]Labarca G, Uribe JP, Pacheco C, et al. Bronchoscopic lung volume reduction with endobronchial zephyr valves for severe emphysema: a systematic review and meta-analysis. Respiration. 2019 May 22;98(3):268-78.
http://www.ncbi.nlm.nih.gov/pubmed/31117102?tool=bestpractice.com
The procedure may be most beneficial in patients whose dyspnea is primarily due to hyperinflation and air trapping in the air spaces distal to the terminal bronchioles, which manifests as emphysema with markedly increased residual volume. Contraindications include active lung infection and incomplete lobar fissures (<80%).[223]Abia-Trujillo D, Johnson MM, Patel NM, et al. Bronchoscopic lung volume reduction: a new hope for patients with severe emphysema and air trapping. Mayo Clin Proc. 2021 Feb;96(2):464-72.
http://www.ncbi.nlm.nih.gov/pubmed/32829903?tool=bestpractice.com
The most common adverse events associated with endobronchial valve insertion are pneumothorax and exacerbation.[220]van Geffen WH, Slebos DJ, Herth FJ, et al. Surgical and endoscopic interventions that reduce lung volume for emphysema: a systemic review and meta-analysis. Lancet Respir Med. 2019 Apr;7(4):313-24.
http://www.ncbi.nlm.nih.gov/pubmed/30744937?tool=bestpractice.com
]
How does bronchoscopic lung volume reduction compare with medical therapy in people with chronic obstructive pulmonary disease?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1680/fullShow me the answer One meta-analysis found an increased risk of early mortality in patients who underwent lung volume reduction surgery compared to standard care; however, no significant difference was observed in overall mortality.[220]van Geffen WH, Slebos DJ, Herth FJ, et al. Surgical and endoscopic interventions that reduce lung volume for emphysema: a systemic review and meta-analysis. Lancet Respir Med. 2019 Apr;7(4):313-24.
http://www.ncbi.nlm.nih.gov/pubmed/30744937?tool=bestpractice.com
Bullectomy is an option in COPD patients with dyspnea in whom CT reveals huge bullae occupying at least 30% of the hemithorax. Severely poor functional status and severe decrease in FEV₁ (<500 mL) make these options less favorable. Endobronchial valve insertion can produce clinically meaningful improvements in appropriately selected patients with COPD.[220]van Geffen WH, Slebos DJ, Herth FJ, et al. Surgical and endoscopic interventions that reduce lung volume for emphysema: a systemic review and meta-analysis. Lancet Respir Med. 2019 Apr;7(4):313-24.
http://www.ncbi.nlm.nih.gov/pubmed/30744937?tool=bestpractice.com
[221]Klooster K, Slebos DJ, Zoumot Z, et al. Endobronchial valves for emphysema: an individual patient-level reanalysis of randomised controlled trials. BMJ Open Respir Res. 2017 Nov 2;4(1):e000214.
https://bmjopenrespres.bmj.com/content/4/1/e000214
http://www.ncbi.nlm.nih.gov/pubmed/29441206?tool=bestpractice.com
[222]Labarca G, Uribe JP, Pacheco C, et al. Bronchoscopic lung volume reduction with endobronchial zephyr valves for severe emphysema: a systematic review and meta-analysis. Respiration. 2019 May 22;98(3):268-78.
http://www.ncbi.nlm.nih.gov/pubmed/31117102?tool=bestpractice.com
The procedure may be most beneficial in patients whose dyspnea is primarily due to hyperinflation and air trapping in the air spaces distal to the terminal bronchioles, which manifests as emphysema with markedly increased residual volume. Contraindications include active lung infection and incomplete lobar fissures (<80%).[223]Abia-Trujillo D, Johnson MM, Patel NM, et al. Bronchoscopic lung volume reduction: a new hope for patients with severe emphysema and air trapping. Mayo Clin Proc. 2021 Feb;96(2):464-72.
http://www.ncbi.nlm.nih.gov/pubmed/32829903?tool=bestpractice.com
The most common adverse events associated with endobronchial valve insertion are pneumothorax and exacerbation.[220]van Geffen WH, Slebos DJ, Herth FJ, et al. Surgical and endoscopic interventions that reduce lung volume for emphysema: a systemic review and meta-analysis. Lancet Respir Med. 2019 Apr;7(4):313-24.
http://www.ncbi.nlm.nih.gov/pubmed/30744937?tool=bestpractice.com
Criteria for referral for lung transplantation include:[224]Leard LE, Holm AM, Valapour M, et al. Consensus document for the selection of lung transplant candidates: an update from the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2021 Nov;40(11):1349-79.
https://www.jhltonline.org/article/S1053-2498(21)02407-4/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34419372?tool=bestpractice.com
Body mass index, airflow Obstruction, Dyspnea, and Exercise (BODE) score 5-6 with additional factor(s) present suggestive of increased risk of mortality:
Frequent acute exacerbations
Increase in BODE score >1 over past 24 months
Pulmonary artery to aorta diameter >1 on CT scan
FEV₁ 20% to 25% predicted
Clinical deterioration despite maximal treatment including medication, pulmonary rehabilitation, oxygen therapy, and, as appropriate, nocturnal noninvasive positive pressure ventilation
Poor quality of life unacceptable to the patient
For a patient who is a candidate for bronchoscopic or surgical lung volume reduction (LVR), simultaneous referral for both lung transplant and LVR evaluation is appropriate
[
BODE Index for COPD Survival Prediction
Opens in new window
]
Lung transplantation has been shown to improve quality of life and functional capacity.[219]van Agteren JE, Carson KV, Tiong LU, et al. Lung volume reduction surgery for diffuse emphysema. Cochrane Database Syst Rev. 2016 Oct 14;(10):CD001001.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD001001.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/27739074?tool=bestpractice.com
However, lung transplantation does not appear to confer a survival benefit.[225]Stavem K, Bjørtuft Ø, Borgan Ø, et al. Lung transplantation in patients with chronic obstructive pulmonary disease in a national cohort is without obvious survival benefit. J Heart Lung Transplant. 2006 Jan;25(1):75-84.
http://www.ncbi.nlm.nih.gov/pubmed/16399534?tool=bestpractice.com
Palliative care
Palliative therapies to improve symptoms of dyspnea, offer nutritional support, address anxiety and depression, and reduce fatigue may benefit patients with COPD who experience these despite optimal medical therapy. End-of-life care and hospice admission should be considered for patients with very advanced disease. Patient and family should be well educated about the process, and it is suggested that discussions should be held early in the course of the disease before acute respiratory failure develops.[1]Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2025 report. 2025 [internet publication].
https://goldcopd.org/2025-gold-report
[226]Janssen DJA, Bajwah S, Boon MH, et al. European Respiratory Society clinical practice guideline: palliative care for people with COPD or interstitial lung disease. Eur Respir J. 2023 Aug;62(2):2202014.
https://erj.ersjournals.com/content/62/2/2202014
http://www.ncbi.nlm.nih.gov/pubmed/37290789?tool=bestpractice.com
Opioid analgesics, fans, neuromuscular electrical stimulation, and chest wall vibration can relieve dyspnea.[1]Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2025 report. 2025 [internet publication].
https://goldcopd.org/2025-gold-report
One study has suggested that low doses of an opioid analgesic and a benzodiazepine are safe and are not associated with increased hospital admissions or mortality.[227]Ekström MP, Bornefalk-Hermansson A, Abernethy AP, et al. Safety of benzodiazepines and opioids in very severe respiratory disease: national prospective study. BMJ. 2014 Jan 30;348:g445.
https://www.bmj.com/content/348/bmj.g445.long
http://www.ncbi.nlm.nih.gov/pubmed/24482539?tool=bestpractice.com
Another study found that regular, low-dose, oral sustained-release morphine for 4 weeks improved disease-specific health status in patients with COPD and refractory breathlessness.[228]Verberkt CA, van den Beuken-van Everdingen MHJ, Schols JMGA, et al. Effect of sustained-release morphine for refractory breathlessness in chronic obstructive pulmonary disease on health status: a randomized clinical trial. JAMA Intern Med. 2020 Oct 1;180(10):1306-14.
https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2769373
http://www.ncbi.nlm.nih.gov/pubmed/32804188?tool=bestpractice.com
One Cochrane review concluded that there is no evidence for or against benzodiazepines for the relief of breathlessness in people with advanced cancer and COPD.[229]Simon ST, Higginson IJ, Booth S, et al. Benzodiazepines for the relief of breathlessness in advanced malignant and non-malignant diseases in adults. Cochrane Database Syst Rev. 2016 Oct 20;(10):CD007354.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD007354.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/27764523?tool=bestpractice.com
Acupuncture and acupressure may also improve breathlessness and quality of life in patients with advanced COPD.[230]von Trott P, Oei SL, Ramsenthaler C. Acupuncture for breathlessness in advanced diseases: a systematic review and meta-analysis. J Pain Symptom Manage. 2020 Feb;59(2):327-38.e3.
http://www.ncbi.nlm.nih.gov/pubmed/31539602?tool=bestpractice.com
 ]
[Evidence A]
]
[Evidence A]

 ]
Newer LAMAs, such as aclidinium, glycopyrrolate, and umeclidinium, have at least comparable efficacy to tiotropium, in terms of change from baseline in trough FEV₁, transitional dyspnea index focal score, St George's Respiratory Questionnaire score, and rescue medication use.[100] Revefenacin is a nebulized LAMA approved for the maintenance treatment of moderate to severe COPD. There is a suggestion of increased cardiovascular-related mortality in some studies of patients taking short-acting muscarinic antagonists and in some studies of patients taking LAMAs.[101][102] One study concluded that aclidinium was not associated with an increase in major adverse cardiovascular events, compared with placebo.[103] A population-based cohort study found that older men with COPD newly started on LAMAs are at increased risk of urinary tract infections.[104]
]
Newer LAMAs, such as aclidinium, glycopyrrolate, and umeclidinium, have at least comparable efficacy to tiotropium, in terms of change from baseline in trough FEV₁, transitional dyspnea index focal score, St George's Respiratory Questionnaire score, and rescue medication use.[100] Revefenacin is a nebulized LAMA approved for the maintenance treatment of moderate to severe COPD. There is a suggestion of increased cardiovascular-related mortality in some studies of patients taking short-acting muscarinic antagonists and in some studies of patients taking LAMAs.[101][102] One study concluded that aclidinium was not associated with an increase in major adverse cardiovascular events, compared with placebo.[103] A population-based cohort study found that older men with COPD newly started on LAMAs are at increased risk of urinary tract infections.[104] ]
[Evidence A] In cases of stable COPD, if the decision is made to use single-agent therapy, LAMA may be superior to LABA agents.[105] LAMAs have a greater effect on exacerbation reduction than LABAs in patients with moderate to very severe COPD.[106][107] The long-term safety of LAMA was demonstrated in the UPLIFT trial.[108]
]
[Evidence A] In cases of stable COPD, if the decision is made to use single-agent therapy, LAMA may be superior to LABA agents.[105] LAMAs have a greater effect on exacerbation reduction than LABAs in patients with moderate to very severe COPD.[106][107] The long-term safety of LAMA was demonstrated in the UPLIFT trial.[108] ]
]
 ]
]
 ]
One systematic review and network meta-analysis found that all LABA/LAMA fixed-dose combinations had a similar efficacy and safety.[115]
]
One systematic review and network meta-analysis found that all LABA/LAMA fixed-dose combinations had a similar efficacy and safety.[115] ]
This risk is higher for fluticasone in comparison with budesonide.[122][123][124] A study in a large cohort of Danish patients found the risk of acquiring Pseudomonas aeruginosa, a common cause of hospital-acquired pneumonia, to be dose-dependent, with high-dose ICS associated with the greatest risk. The study also found that patients with P aeruginosa were more likely to have a lower BMI and FEV₁ than P aeruginosa-negative patients.[125] One systematic review and meta-analysis found that, despite a significant increase in unadjusted risk of pneumonia associated with use of ICS, pneumonia fatality and overall mortality were not increased in randomized controlled trials and were decreased in observational studies.[126] Therefore, an individualized treatment approach that assesses a patient's risk of pneumonia versus the benefit of decreased exacerbations should be implemented.[85][127][128] Concern is also raised with regards to increased risk of tuberculosis and influenza in adult patients with COPD who are on ICS therapy, although one meta-analysis found that less than 1% of all assessed tuberculosis cases were attributable to ICS exposure.[129][130] ICS may also cause oropharyngeal candidiasis and hoarseness.[85]
[
]
This risk is higher for fluticasone in comparison with budesonide.[122][123][124] A study in a large cohort of Danish patients found the risk of acquiring Pseudomonas aeruginosa, a common cause of hospital-acquired pneumonia, to be dose-dependent, with high-dose ICS associated with the greatest risk. The study also found that patients with P aeruginosa were more likely to have a lower BMI and FEV₁ than P aeruginosa-negative patients.[125] One systematic review and meta-analysis found that, despite a significant increase in unadjusted risk of pneumonia associated with use of ICS, pneumonia fatality and overall mortality were not increased in randomized controlled trials and were decreased in observational studies.[126] Therefore, an individualized treatment approach that assesses a patient's risk of pneumonia versus the benefit of decreased exacerbations should be implemented.[85][127][128] Concern is also raised with regards to increased risk of tuberculosis and influenza in adult patients with COPD who are on ICS therapy, although one meta-analysis found that less than 1% of all assessed tuberculosis cases were attributable to ICS exposure.[129][130] ICS may also cause oropharyngeal candidiasis and hoarseness.[85]
[  ]
]
 ]
This is convenient and may help with compliance in some patients. The choice of therapy in this class is based on availability and individual response and preference.[133] The combination may be provided in separate inhalers or a combination inhaler.
]
This is convenient and may help with compliance in some patients. The choice of therapy in this class is based on availability and individual response and preference.[133] The combination may be provided in separate inhalers or a combination inhaler.  ]
]
 ]
There are no data showing the efficacy or safety of chronic azithromycin treatment beyond 1 year of treatment.
]
There are no data showing the efficacy or safety of chronic azithromycin treatment beyond 1 year of treatment.  ]
The optimal timing, components, duration, and models for improving physical activity remain unclear. Meta-analyses suggest that yoga, Qigong, and other home-based breathing exercises can improve exercise capacity and pulmonary function in patients with COPD.[179][180][181] Tai Chi has been shown to improve exercise capacity compared with usual care.[182]
]
The optimal timing, components, duration, and models for improving physical activity remain unclear. Meta-analyses suggest that yoga, Qigong, and other home-based breathing exercises can improve exercise capacity and pulmonary function in patients with COPD.[179][180][181] Tai Chi has been shown to improve exercise capacity compared with usual care.[182] ]
They result in a small reduction in the frequency of acute exacerbations and in days of disability per month, but do not improve lung function or quality of life.[190][191][192] Erdosteine and carbocysteine are not available in the US. Treatment with mucolytic agents such as carbocysteine and acetylcysteine may reduce exacerbations and modestly improve health status in patients not receiving ICS.[1] However, erdosteine may have a significant effect on mild exacerbations whether or not the patient is taking ICS.[1]
]
They result in a small reduction in the frequency of acute exacerbations and in days of disability per month, but do not improve lung function or quality of life.[190][191][192] Erdosteine and carbocysteine are not available in the US. Treatment with mucolytic agents such as carbocysteine and acetylcysteine may reduce exacerbations and modestly improve health status in patients not receiving ICS.[1] However, erdosteine may have a significant effect on mild exacerbations whether or not the patient is taking ICS.[1] ]
There is evidence to support starting pulmonary rehabilitation within 1 month of an acute exacerbation.[196][197]
]
There is evidence to support starting pulmonary rehabilitation within 1 month of an acute exacerbation.[196][197] ]
]
 ]
They are used to improve lung dynamics, exercise adherence, and quality of life.[219] Lung volume reduction surgery is indicated in patients with very severe airflow limitation, and especially in patients with localized upper lobe disease and lower than normal exercise capacity.[218]
[
]
They are used to improve lung dynamics, exercise adherence, and quality of life.[219] Lung volume reduction surgery is indicated in patients with very severe airflow limitation, and especially in patients with localized upper lobe disease and lower than normal exercise capacity.[218]
[  ]
One meta-analysis found an increased risk of early mortality in patients who underwent lung volume reduction surgery compared to standard care; however, no significant difference was observed in overall mortality.[220] Bullectomy is an option in COPD patients with dyspnea in whom CT reveals huge bullae occupying at least 30% of the hemithorax. Severely poor functional status and severe decrease in FEV₁ (<500 mL) make these options less favorable. Endobronchial valve insertion can produce clinically meaningful improvements in appropriately selected patients with COPD.[220][221][222] The procedure may be most beneficial in patients whose dyspnea is primarily due to hyperinflation and air trapping in the air spaces distal to the terminal bronchioles, which manifests as emphysema with markedly increased residual volume. Contraindications include active lung infection and incomplete lobar fissures (<80%).[223] The most common adverse events associated with endobronchial valve insertion are pneumothorax and exacerbation.[220]
]
One meta-analysis found an increased risk of early mortality in patients who underwent lung volume reduction surgery compared to standard care; however, no significant difference was observed in overall mortality.[220] Bullectomy is an option in COPD patients with dyspnea in whom CT reveals huge bullae occupying at least 30% of the hemithorax. Severely poor functional status and severe decrease in FEV₁ (<500 mL) make these options less favorable. Endobronchial valve insertion can produce clinically meaningful improvements in appropriately selected patients with COPD.[220][221][222] The procedure may be most beneficial in patients whose dyspnea is primarily due to hyperinflation and air trapping in the air spaces distal to the terminal bronchioles, which manifests as emphysema with markedly increased residual volume. Contraindications include active lung infection and incomplete lobar fissures (<80%).[223] The most common adverse events associated with endobronchial valve insertion are pneumothorax and exacerbation.[220]






