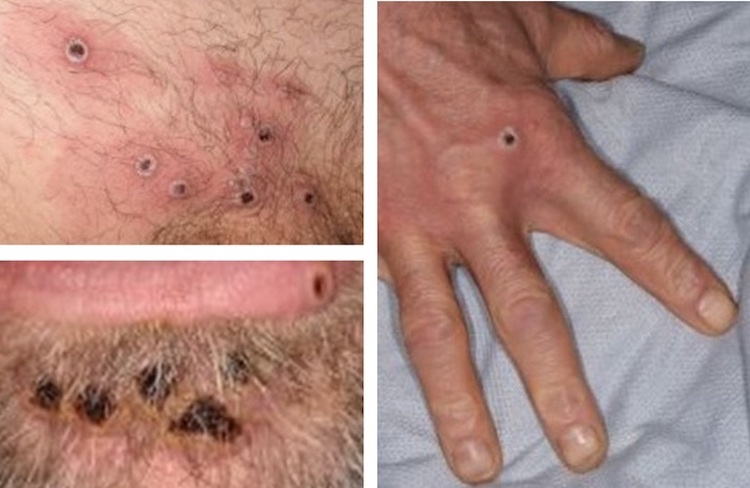
WHO declares mpox emergency over
The World Health Organization (WHO) declared that the mpox public health emergency of international concern (PHEIC) was over on 5 September 2025.[13] The PHEIC was first declared in August 2024 due to various mpox outbreaks in Africa.
The decision to end the PHEIC is based on a sustained decline in the number of cases and deaths in the Democratic Republic of the Congo (DRC), and other affected countries including Uganda, Sierra Leone, and Burundi, as well as the fact that there is now a better understanding of the drivers of transmission and the risk factors for disease severity. Despite lifting the PHEIC, the WHO’s mpox response will continue.[25]
In the past 12 months, approximately 43,800 laboratory-confirmed cases of mpox have been reported in Africa, including 186 deaths (as of 28 September 2025). The three countries with the majority of cases were the DRC, Uganda, and Sierra Leone.[13] The spread in Africa was due to two distinct outbreaks:
A clade Ia outbreak primarily in mpox-endemic areas and mainly affecting children.
A clade Ib outbreak in the eastern part of the country that spread rapidly and reached neighboring countries that had not previously reported cases.
The WHO has assessed the overall global public health risk to be moderate for clade Ib and clade IIa mpox, and low for clade Ia and IIb mpox.[13]
This was the second time that the WHO declared an mpox outbreak to constitute a PHEIC, with the first one declared in July 2022 due to a global outbreak of clade IIb mpox in countries that had not previously experienced cases. The emergency was declared over in May 2023 as the number of cases had decreased significantly since their peak in August 2022. However, clade II mpox is still circulating globally with outbreaks in many countries.
Summary
Definition
History and exam
Other diagnostic factors
- fatigue/asthenia/malaise
- myalgia
- headache
- sore throat
- backache
- cough
- nausea/vomiting
- diarrhea
- delirium/confusion
- seizures
Risk factors
- recent travel to/living in endemic country or country with outbreak
- contact with suspected, probable, or confirmed case
- occupational exposure
- unprotected sexual encounters or multiple sexual partners
- recent tattoo or piercing
- contact with infected animal
- children (severe disease)
- pregnant women (severe disease)
- immunocompromised (severe disease)
- HIV infection (severe disease)
- sexually transmitted infection (STI)
- acute or chronic skin conditions (severe disease)
Diagnostic tests
1st tests to order
- reverse-transcription polymerase chain reaction (RT-PCR)
- CBC
- blood urea nitrogen and electrolytes
- LFTs
- sexually transmitted infection (STI) tests
Treatment algorithm
Contributors
Authors
David L. Heymann, MD, DTM&H
Professor of Infectious Disease Epidemiology
London School of Hygiene and Tropical Medicine
University of London
Head
Centre on Global Health Security - Chatham House
London
UK
Disclosures
DLH declares that he has no competing interests.
Acknowledgements
Dr David L. Heymann would like to gratefully acknowledge Dr Tom Blanchard, the previous contributor to this topic.
Disclosures
TB is the principal investigator on an MRC/Wellcome/Newton Fund grant to make a Zika vaccine based on recombinant modified vaccinia Ankara.
Peer reviewers
Miguel G. Madariaga, MD, MSc, FACP
Infectious Diseases Consultant
Naples Community Hospital
Naples
FL
Disclosures
MGM declares that he has no competing interests.
Jimmy Whitworth, MD, FRCP, FFPH, FMedSci, DTM&H
Emeritus Professor
London School of Hygiene & Tropical Medicine
London
UK
Disclosures
JW declares that he has no competing interests.
Peer reviewer acknowledgements
BMJ Best Practice topics are updated on a rolling basis in line with developments in evidence and guidance. The peer reviewers listed here have reviewed the content at least once during the history of the topic.
Disclosures
Peer reviewer affiliations and disclosures pertain to the time of the review.
References
Key articles
World Health Organization. Clinical management and infection prevention and control for mpox: living guideline, May 2025. Jun 2025 [internet publication].Full text
Centers for Disease Control and Prevention. Clinical overview of monkeypox. Apr 2025 [internet publication].Full text
World Health Organization. Surveillance, case investigation and contact tracing for mpox: interim guidance, 27 November 2024. Dec 2024 [internet publication].Full text
Centers for Disease Control and Prevention. Clinical treatment of monkeypox. Aug 2025 [internet publication].Full text
World Health Organization. Diagnostic testing for the monkeypox virus (MPXV): interim guidance. May 2024 [internet publication].Full text
Centers for Disease Control and Prevention. Clinical considerations for pain management. Sep 2024 [internet publication].Full text
Reference articles
A full list of sources referenced in this topic is available here.
Use of this content is subject to our disclaimer