Ebola disease is a notifiable disease. The case definition is very broad and includes a long list of possible differential diagnoses.
The initial assessment of a patient with suspected Ebola disease hinges on two main factors:
Epidemiologic risk (e.g., living or working in, or travel to, endemic area in previous 21 days); and
Presence or history of a fever in the past 24 hours.
Infection prevention and control
Infection prevention and control (IPC) is of immediate concern and local protocols should be followed. Infection risk should be assessed. Having determined that a patient may be infected, the physician needs to determine how infectious the patient is currently. For example, the absence of vomiting/diarrhea reduces the risk; however, uncontrolled diarrhea greatly increases the risk of transmission.
Identifying that a symptomatic patient may be at risk of infection mandates precautionary isolation procedures and use of personal protective equipment (PPE) until the infection is either confirmed or excluded. It is extremely important to minimize the risk of transmission while working up the patient.[93]Fletcher TE, Brooks TJ, Beeching NJ. Ebola and other viral haemorrhagic fevers. BMJ. 2014 Aug 11;349:g5079.
https://www.bmj.com/content/349/bmj.g5079
http://www.ncbi.nlm.nih.gov/pubmed/25113010?tool=bestpractice.com
The World Health Organization (WHO) recommends the following IPC principles in healthcare settings.[94]World Health Organization. Infection prevention and control guideline for Ebola and Marburg disease. Aug 2023 [internet publication].
https://www.who.int/publications/i/item/WHO-WPE-CRS-HCR-2023.1
An IPC ring approach is recommended in healthcare facilities and communities during the management of cases
In geographic areas where the virus is circulating, all people should be screened at the first point of contact with a healthcare facility, using a no-touch technique, to enable early recognition of suspected cases.
All suspected cases should be triaged to determine the severity of disease and identify patients in need of immediate care.
Patients with suspected or confirmed infection should be isolated, preferably in a single room.
Hand hygiene should be performed using an alcohol-based hand rub or soap and running water using the correct technique.
Appropriate PPE should be worn when in contact with a suspected or confirmed case.
Mucous membranes of the eyes, mouth, and nose should be completely covered. A face shield or goggles (under the head-and-neck covering) should be used. Fluid-resistant surgical or medical masks with a structured design (so that they do not collapse against the mouth) are recommended. A fluid-resistant particulate respirator should be used during aerosol-generating procedures.
Gloves and a disposable gown (or coverall) and apron made of fabric that has been tested for resistance to penetration by body fluids or blood-borne pathogens should be worn. Nitrile gloves are preferred over latex gloves.
Specific PPE requirements depend on the level of patient contact (i.e., indirect contact such as screening and triage versus direct contact of a case). PPE is not required during screening activities where a distance of at least 1 meter can be guaranteed and a no-touch approach is strictly followed.
Surfaces should be disinfected (using the wiping method) in facilities and settings that provide care to patients with suspected or confirmed infection.
All waste generated from the care of a patient with suspected or confirmed infection should be treated as infectious waste.
Healthcare workers with occupational exposure should be immediately assessed for exposure risk and managed accordingly.
More detailed IPC guidance is available from the WHO:
Key infection prevention and control measures in the World Health Organisation (WHO) guideline for Ebola. Willet V et al. BMJ 2024; 384 :p2811 doi:10.1136/bmj.p2811; used with permission
Opens in new window
Guidance is also available from the CDC:
History
A detailed history helps to clarify the level of risk for infection, as well as assess the possibility of other causes of an acute febrile syndrome.
People living or working in endemic areas (e.g., West Africa, Democratic Republic of the Congo) are at high risk of infection. However, recent arrival from endemic areas is also an important risk factor.
Most patients with suspected infection in developed countries will be returning travelers and healthcare workers who have cared for patients during outbreaks. Therefore, a comprehensive travel history is extremely important. History of recent arrival from an endemic area is significant. Up-to-date knowledge of the geographic locations of active epidemics helps to clarify the patient’s epidemiologic risk.
Apart from healthcare workers, other high-risk occupations include those where people work with primates or bats from endemic areas, or high-risk clinical samples.
As malaria is still the most common cause of febrile illness in returning travelers from West Africa, the presence of risk factors for acquiring malaria should be assessed (e.g., living/working in, or traveling to, endemic area; inadequate or absent chemoprophylaxis; not using insecticides or bed nets).[95]Mendelson M, Han PV, Vincent P, et al. Regional variation in travel-related illness acquired in Africa, March 1997-May 2011. Emerg Infect Dis. 2014 Apr;20(4):532-41.
https://wwwnc.cdc.gov/eid/article/20/4/13-1128_article
http://www.ncbi.nlm.nih.gov/pubmed/24655358?tool=bestpractice.com
However, coinfection with malaria was seen in up to 5% of patients in West Africa during the 2014 outbreak, so the possibility of dual infection should be considered in all patients.[96]Hunt L, Gupta-Wright A, Simms V, et al. Clinical presentation, biochemical, and haematological parameters and their association with outcome in patients with Ebola virus disease: an observational cohort study. Lancet Infect Dis. 2015 Nov;15(11):1292-9.
http://www.ncbi.nlm.nih.gov/pubmed/26271406?tool=bestpractice.com
Exposure risk
Contacts of infected patients (including healthcare workers and household contacts) are at risk of infection if the person was exposed to body fluids of the infected patient without appropriate protective equipment. The incubation period after infection is 2-21 days.[3]World Health Organization. Ebola virus disease fact sheet. Apr 2023 [internet publication].
https://www.who.int/news-room/fact-sheets/detail/ebola-virus-disease
Incubation periods may be shorter in children.[76]WHO Ebola Response Team. Ebola virus disease among children in West Africa. N Engl J Med. 2015 Mar 26;372(13):1274-7.
https://www.nejm.org/doi/full/10.1056/NEJMc1415318
http://www.ncbi.nlm.nih.gov/pubmed/25806936?tool=bestpractice.com
Brief interactions, such as walking by a person or moving through a hospital, do not constitute close contact.
Contact is defined by the WHO as someone who has:[97]World Health Organization. Case definition recommendations for Ebola or Marburg virus diseases. August 2014 [internet publication].
https://apps.who.int/iris/handle/10665/146397
Slept in the same household as a patient
Had direct physical contact with the patient during the illness or at the funeral
Touched the patient's body fluids or clothes/bed linens during the illness
Been breastfed by the patient (babies).
Case definitions
Case definitions differ depending on the organization. Links to the case definitions by the CDC and WHO are below:
Symptoms
Patients are not considered infectious until they develop symptoms. The initial presentation is nonspecific, which makes early clinical diagnosis difficult; however, typical symptoms include:[98]Centers for Disease Control and Prevention. Ebola virus disease fact sheet. May 2024 [internet publication].
https://www.cdc.gov/ebola/hcp/communication-resources/ebola-virus-disease-factsheet.html
[99]Centers for Disease Control and Prevention. Clinical signs of Ebola disease. Jan 2025 [internet publication].
https://www.cdc.gov/ebola/hcp/clinical-signs/index.html
The most common symptoms reported on admission during the 2014 outbreak were: fever (76%), fatigue (71%), anorexia (64%), headache (56%), diarrhea (51%), vomiting (50%), myalgia/arthralgia (48%), abdominal pain (40%), sore throat (29%), and conjunctivitis (27%). Other less common symptoms included difficulty swallowing (22%), difficulty breathing (18%), hiccups (13%), hemorrhagic signs (11%), confusion (9%), and rash (3%).[100]Rojek AM, Salam A, Ragotte RJ, et al. A systematic review and meta-analysis of patient data from the West Africa (2013-16) Ebola virus disease epidemic. Clin Microbiol Infect. 2019 Nov;25(11):1307-14.
https://www.doi.org/10.1016/j.cmi.2019.06.032
http://www.ncbi.nlm.nih.gov/pubmed/31284032?tool=bestpractice.com
Three phases of illness are typically recognized, starting with a few days of nonspecific fever, headache, and myalgia, and followed by a gastrointestinal phase where diarrhea, vomiting, abdominal symptoms, and dehydration are prominent.[64]Sharma N, Cappell MS. Gastrointestinal and hepatic manifestations of Ebola virus infection. Dig Dis Sci. 2015 Sep;60(9):2590-603.
http://www.ncbi.nlm.nih.gov/pubmed/25972150?tool=bestpractice.com
In the second week, the patient may either recover, or deteriorate with a third phase of illness, which includes collapse, neurologic manifestations, and bleeding. This phase is often fatal.[18]WHO Ebola Response Team. Ebola virus disease in West Africa: the first 9 months of the epidemic and forward projections. N Engl J Med. 2014 Oct 16;371(16):1481-95.
https://www.nejm.org/doi/full/10.1056/NEJMoa1411100#t=article
http://www.ncbi.nlm.nih.gov/pubmed/25244186?tool=bestpractice.com
Data from the 2014 outbreak indicate that children are relatively spared; however, this may be confounded by a high fatality rate before being registered as a case, or the bias of high rates in healthcare workers.[101]Glynn JR. Age-specific incidence of Ebola virus disease. Lancet. 2015 Aug 1;386(9992):432.
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(15)61446-5/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/26251391?tool=bestpractice.com
Children present with similar symptoms to adults; however, in previous outbreaks, younger children are reported to have more respiratory (e.g., cough, dyspnea) and gastrointestinal symptoms, but less bleeding and neurologic signs compared with adults.[102]Mupere E, Kaducu OF, Yoti Z. Ebola haemorrhagic fever among hospitalised children and adolescents in northern Uganda: epidemiologic and clinical observations. Afr Health Sci. 2001 Dec;1(2):60-5.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2141551
http://www.ncbi.nlm.nih.gov/pubmed/12789118?tool=bestpractice.com
Data were sparse for this patient group in the 2014 outbreak.[102]Mupere E, Kaducu OF, Yoti Z. Ebola haemorrhagic fever among hospitalised children and adolescents in northern Uganda: epidemiologic and clinical observations. Afr Health Sci. 2001 Dec;1(2):60-5.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2141551
http://www.ncbi.nlm.nih.gov/pubmed/12789118?tool=bestpractice.com
[103]Peacock G, Uyeki TM, Rasmussen SA. Ebola virus disease and children: what pediatric health care professionals need to know. JAMA Pediatr. 2014 Dec;168(12):1087-8.
https://jamanetwork.com/journals/jamapediatrics/fullarticle/1918461
http://www.ncbi.nlm.nih.gov/pubmed/25325785?tool=bestpractice.com
A pediatric cohort study in Sierra Leone described symptoms in 282 patients and found vomiting (60%), abdominal pain (59%), diarrhea (45%), and conjunctivitis (38%) were common, while hiccups (5%) and bleeding (2%) were rare.[104]Fitzgerald F, Naveed A, Wing K, et al. Ebola virus disease in children, Sierra Leone, 2014-2015. Emerg Infect Dis. 2016 Oct;22(10):1769-77.
https://wwwnc.cdc.gov/eid/article/22/10/16-0579_article
http://www.ncbi.nlm.nih.gov/pubmed/27649367?tool=bestpractice.com
Another study in Sierra Leone found that weakness, fever, and distress were each present in more than 63% of children, and loss of appetite, diarrhea, and cough were present in more than 50%. Approximately 25% of these children did not have fever at the time of admission.[105]Shah T, Greig J, van der Plas LM, et al. Inpatient signs and symptoms and factors associated with death in children aged 5 years and younger admitted to two Ebola management centres in Sierra Leone, 2014: a retrospective cohort study. Lancet Glob Health. 2016 Jul;4(7):e495-501.
https://www.thelancet.com/journals/langlo/article/PIIS2214-109X(16)30097-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/27340004?tool=bestpractice.com
Anecdotally, children under 4 years of age initially present with more subtle symptoms before developing a fever, and are often diagnosed later in the course of illness.
Physical exam
A full physical exam should be undertaken with the aim of excluding a focus for sepsis while looking for signs of viral hemorrhagic fever (e.g., conjunctival injection, purpuric rash, or other signs of bleeding).
Vital signs should be taken:
Fever: the presenting symptom in up to 90% of patients, its presence is enough to raise concern for infection in the appropriate epidemiologic context.[18]WHO Ebola Response Team. Ebola virus disease in West Africa: the first 9 months of the epidemic and forward projections. N Engl J Med. 2014 Oct 16;371(16):1481-95.
https://www.nejm.org/doi/full/10.1056/NEJMoa1411100#t=article
http://www.ncbi.nlm.nih.gov/pubmed/25244186?tool=bestpractice.com
[22]Schieffelin JS, Shaffer JG, Goba A, et al; KGH Lassa Fever Program; Viral Hemorrhagic Fever Consortium; WHO Clinical Response Team. Clinical illness and outcomes in patients with Ebola in Sierra Leone. N Engl J Med. 2014 Nov 27;371(22):2092-100.
https://www.nejm.org/doi/full/10.1056/NEJMoa1411680#t=article
http://www.ncbi.nlm.nih.gov/pubmed/25353969?tool=bestpractice.com
[106]Dananché C, Bénet T, Vanhems P. Ebola: fever definitions might delay detection in non-epidemic areas. Lancet. 2014 Dec;168(12):1087-8.
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(14)61787-6/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/25455239?tool=bestpractice.com
Although fever is a major presenting symptom, a normal temperature at presentation is common. Wide variations in body temperature can be observed during the course of illness, especially in children, with normothermia or hypothermia occurring in the later stages of fatal infection.[16]Kortepeter MG, Bausch DG, Bray M. Basic clinical and laboratory features of filoviral hemorrhagic fever. J Infect Dis. 2011 Nov;204 Suppl 3:S810-6
https://jid.oxfordjournals.org/content/204/suppl_3/S810.long
http://www.ncbi.nlm.nih.gov/pubmed/21987756?tool=bestpractice.com
[17]Bwaka MA, Bonnet MJ, Calain P, et al. Ebola hemorrhagic fever in Kikwit, Democratic Republic of the Congo: clinical observations in 103 patients. J Infect Dis. 1999 Feb;179 Suppl 1:S1-7.
https://jid.oxfordjournals.org/content/179/Supplement_1/S1.long
http://www.ncbi.nlm.nih.gov/pubmed/9988155?tool=bestpractice.com
[102]Mupere E, Kaducu OF, Yoti Z. Ebola haemorrhagic fever among hospitalised children and adolescents in northern Uganda: epidemiologic and clinical observations. Afr Health Sci. 2001 Dec;1(2):60-5.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2141551
http://www.ncbi.nlm.nih.gov/pubmed/12789118?tool=bestpractice.com
[105]Shah T, Greig J, van der Plas LM, et al. Inpatient signs and symptoms and factors associated with death in children aged 5 years and younger admitted to two Ebola management centres in Sierra Leone, 2014: a retrospective cohort study. Lancet Glob Health. 2016 Jul;4(7):e495-501.
https://www.thelancet.com/journals/langlo/article/PIIS2214-109X(16)30097-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/27340004?tool=bestpractice.com
[107]Lado M, Howlett P. Ebola virus disease in children: towards a better clinical picture and improved management. Lancet Glob Health. 2016 Jul;4(7):e436-7.
https://www.thelancet.com/journals/langlo/article/PIIS2214-109X(16)30111-5/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/27339995?tool=bestpractice.com
Some patients may initially have a low-grade fever with no other symptoms, or alternatively the temperature may be near normal at first evaluation.[108]Lópaz MA, Amela C, Ordobas M, et al; Working group of Ebola outbreak investigation team of Madrid. First secondary case of Ebola outside Africa: epidemiological characteristics and contact monitoring, Spain, September to November 2014. Euro Surveill. 2015 Jan 8;20(1):21003.
https://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=21003
The temperature threshold for fever differs among countries and guidelines, and using a lower temperature threshold (e.g., ≥99.5°F [37.5°C]) increases the sensitivity of finding cases.[106]Dananché C, Bénet T, Vanhems P. Ebola: fever definitions might delay detection in non-epidemic areas. Lancet. 2014 Dec;168(12):1087-8.
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(14)61787-6/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/25455239?tool=bestpractice.com
[109]Lyon GM, Mehta AK, Varkey JB, et al; Emory Serious Communicable Diseases Unit. Clinical care of two patients with Ebola virus disease in the United States. N Engl J Med. 2014 Dec 18;371(25):2402-9.
https://www.nejm.org/doi/full/10.1056/NEJMoa1409838#t=article
http://www.ncbi.nlm.nih.gov/pubmed/25390460?tool=bestpractice.com
The World Health Organization use a threshold of >100.4°F (38°C).[110]World Health Organization. Exit screening at airports, ports and land crossings: interim guidance for Ebola virus disease. November 2014 [internet publication].
https://apps.who.int/iris/bitstream/handle/10665/139691/WHO_EVD_Guidance_PoE_14.2_eng.pdf
However, in a large cohort in Sierra Leone, <30% had a fever of ≥100.4°F (38°C) at presentation, although a history of fever was reported by 89% of patients.[22]Schieffelin JS, Shaffer JG, Goba A, et al; KGH Lassa Fever Program; Viral Hemorrhagic Fever Consortium; WHO Clinical Response Team. Clinical illness and outcomes in patients with Ebola in Sierra Leone. N Engl J Med. 2014 Nov 27;371(22):2092-100.
https://www.nejm.org/doi/full/10.1056/NEJMoa1411680#t=article
http://www.ncbi.nlm.nih.gov/pubmed/25353969?tool=bestpractice.com
Blood pressure: hypotension is a feature of preterminal disease and shock. It is under-documented in field studies, owing to a lack of measuring equipment in endemic areas.[16]Kortepeter MG, Bausch DG, Bray M. Basic clinical and laboratory features of filoviral hemorrhagic fever. J Infect Dis. 2011 Nov;204 Suppl 3:S810-6
https://jid.oxfordjournals.org/content/204/suppl_3/S810.long
http://www.ncbi.nlm.nih.gov/pubmed/21987756?tool=bestpractice.com
However, septic shock with vascular leakage and microcirculatory failure does not appear to be a dominant feature.
Pulse rate: bradycardia may be present in the initial stages of illness; however, tachycardia may be seen in the later stages of fatal infections.[16]Kortepeter MG, Bausch DG, Bray M. Basic clinical and laboratory features of filoviral hemorrhagic fever. J Infect Dis. 2011 Nov;204 Suppl 3:S810-6
https://jid.oxfordjournals.org/content/204/suppl_3/S810.long
http://www.ncbi.nlm.nih.gov/pubmed/21987756?tool=bestpractice.com
Respiratory rate: tachypnea, along with tachycardia, correlates with a more severe or advanced infection, and is more likely to be respiratory compensation of a metabolic acidosis rather than respiratory involvement.[16]Kortepeter MG, Bausch DG, Bray M. Basic clinical and laboratory features of filoviral hemorrhagic fever. J Infect Dis. 2011 Nov;204 Suppl 3:S810-6
https://jid.oxfordjournals.org/content/204/suppl_3/S810.long
http://www.ncbi.nlm.nih.gov/pubmed/21987756?tool=bestpractice.com
However, respiratory involvement has been described.[111]Petrosillo N, Nicastri E, Lanini S, et al. Ebola virus disease complicated with viral interstitial pneumonia: a case report. BMC Infect Dis. 2015 Oct 16;15:432.
https://www.biomedcentral.com/1471-2334/15/432
http://www.ncbi.nlm.nih.gov/pubmed/26471197?tool=bestpractice.com
Other findings may include:[16]Kortepeter MG, Bausch DG, Bray M. Basic clinical and laboratory features of filoviral hemorrhagic fever. J Infect Dis. 2011 Nov;204 Suppl 3:S810-6
https://jid.oxfordjournals.org/content/204/suppl_3/S810.long
http://www.ncbi.nlm.nih.gov/pubmed/21987756?tool=bestpractice.com
Maculopapular rash: develops early in the course of illness. It is frequently described as nonpruritic, erythematous, and maculopapular. It may begin focally, then become diffuse, generalized, and confluent. Some have described it as morbilliform. It may become purpuric or petechial later on in the infection in patients with coagulopathy.[23]Nkoghe D, Leroy EM, Toung-Mve M, et al. Cutaneous manifestations of filovirus infections. Int J Dermatol. 2012 Sep;51(9):1037-43.
http://www.ncbi.nlm.nih.gov/pubmed/22909355?tool=bestpractice.com
May be difficult to discern in dark-skinned patients.
Bleeding: bleeding manifestations (e.g., epistaxis, bleeding gums, hemoptysis, easy bruising, conjunctival bleeding, hematuria, oozing from injection or venipuncture sites) were present in 30% to 36% of infected patients in previous outbreaks; however, they were reported in fewer patients in more recent outbreaks.[8]Barrette RW, Metwally SA, Rowland JM, et al. Discovery of swine as a host for the Reston ebolavirus. Science. 2009 Jul 10;325(5937):204-6.
https://science.sciencemag.org/content/325/5937/204.full
http://www.ncbi.nlm.nih.gov/pubmed/19590002?tool=bestpractice.com
[16]Kortepeter MG, Bausch DG, Bray M. Basic clinical and laboratory features of filoviral hemorrhagic fever. J Infect Dis. 2011 Nov;204 Suppl 3:S810-6
https://jid.oxfordjournals.org/content/204/suppl_3/S810.long
http://www.ncbi.nlm.nih.gov/pubmed/21987756?tool=bestpractice.com
[17]Bwaka MA, Bonnet MJ, Calain P, et al. Ebola hemorrhagic fever in Kikwit, Democratic Republic of the Congo: clinical observations in 103 patients. J Infect Dis. 1999 Feb;179 Suppl 1:S1-7.
https://jid.oxfordjournals.org/content/179/Supplement_1/S1.long
http://www.ncbi.nlm.nih.gov/pubmed/9988155?tool=bestpractice.com
[18]WHO Ebola Response Team. Ebola virus disease in West Africa: the first 9 months of the epidemic and forward projections. N Engl J Med. 2014 Oct 16;371(16):1481-95.
https://www.nejm.org/doi/full/10.1056/NEJMoa1411100#t=article
http://www.ncbi.nlm.nih.gov/pubmed/25244186?tool=bestpractice.com
[19]Dallatomasinas S, Crestani R, Squire JS, et al. Ebola outbreak in rural West Africa: epidemiology, clinical features and outcomes. Trop Med Int Health. 2015 Apr;20(4):448-54.
http://www.ncbi.nlm.nih.gov/pubmed/25565430?tool=bestpractice.com
[20]Bah EI, Lamah MC, Fletcher T, et al. Clinical presentation of patients with Ebola virus disease in Conakry, Guinea. N Engl J Med. 2015 Jan 1;372(1):40-7.
https://www.nejm.org/doi/full/10.1056/NEJMoa1411249#t=article
http://www.ncbi.nlm.nih.gov/pubmed/25372658?tool=bestpractice.com
[21]Chertow DS, Kleine C, Edwards JK, et al. Ebola virus disease in West Africa - clinical manifestations and management. N Engl J Med. 2014 Nov 27;371(22):2054-7.
https://www.nejm.org/doi/full/10.1056/NEJMp1413084
http://www.ncbi.nlm.nih.gov/pubmed/25372854?tool=bestpractice.com
[22]Schieffelin JS, Shaffer JG, Goba A, et al; KGH Lassa Fever Program; Viral Hemorrhagic Fever Consortium; WHO Clinical Response Team. Clinical illness and outcomes in patients with Ebola in Sierra Leone. N Engl J Med. 2014 Nov 27;371(22):2092-100.
https://www.nejm.org/doi/full/10.1056/NEJMoa1411680#t=article
http://www.ncbi.nlm.nih.gov/pubmed/25353969?tool=bestpractice.com
It is less common in children.[104]Fitzgerald F, Naveed A, Wing K, et al. Ebola virus disease in children, Sierra Leone, 2014-2015. Emerg Infect Dis. 2016 Oct;22(10):1769-77.
https://wwwnc.cdc.gov/eid/article/22/10/16-0579_article
http://www.ncbi.nlm.nih.gov/pubmed/27649367?tool=bestpractice.com
Hiccups: a sign of advanced infection, typically seen in the last 2 to 3 days of fatal infections. They are less common in children.[104]Fitzgerald F, Naveed A, Wing K, et al. Ebola virus disease in children, Sierra Leone, 2014-2015. Emerg Infect Dis. 2016 Oct;22(10):1769-77.
https://wwwnc.cdc.gov/eid/article/22/10/16-0579_article
http://www.ncbi.nlm.nih.gov/pubmed/27649367?tool=bestpractice.com
[105]Shah T, Greig J, van der Plas LM, et al. Inpatient signs and symptoms and factors associated with death in children aged 5 years and younger admitted to two Ebola management centres in Sierra Leone, 2014: a retrospective cohort study. Lancet Glob Health. 2016 Jul;4(7):e495-501.
https://www.thelancet.com/journals/langlo/article/PIIS2214-109X(16)30097-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/27340004?tool=bestpractice.com
Hepatomegaly: tender hepatomegaly with the edge of the liver palpable below the ribcage has been reported, but is uncommon.
Lymphadenopathy: enlarged lymph nodes have been reported, but are uncommon.
Neurologic signs: depressed consciousness, encephalopathy, and seizures are rare but their presence indicates advanced infection. Confusion may be multifactorial in children and is associated with a poor prognosis.[105]Shah T, Greig J, van der Plas LM, et al. Inpatient signs and symptoms and factors associated with death in children aged 5 years and younger admitted to two Ebola management centres in Sierra Leone, 2014: a retrospective cohort study. Lancet Glob Health. 2016 Jul;4(7):e495-501.
https://www.thelancet.com/journals/langlo/article/PIIS2214-109X(16)30097-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/27340004?tool=bestpractice.com
[107]Lado M, Howlett P. Ebola virus disease in children: towards a better clinical picture and improved management. Lancet Glob Health. 2016 Jul;4(7):e436-7.
https://www.thelancet.com/journals/langlo/article/PIIS2214-109X(16)30111-5/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/27339995?tool=bestpractice.com
Initial investigations
All individuals who meet the case definition of Ebola disease should be tested. Diagnostic testing is not recommended for asymptomatic individuals or contacts as the likelihood of detecting RNA is very low prior to symptom onset.[112]World Health Organization. Diagnostic testing for Ebola and Marburg virus diseases: interim guidance, 20 December 2024. Jan 2025 [internet publication].
https://www.who.int/publications/i/item/B09221
See Criteria for case definitions.
The recommended sample type for testing is whole blood or plasma. Oral or buccal swabs are not recommended in patients who are alive.[112]World Health Organization. Diagnostic testing for Ebola and Marburg virus diseases: interim guidance, 20 December 2024. Jan 2025 [internet publication].
https://www.who.int/publications/i/item/B09221
All specimens should be collected according to strict protocols. The CDC and WHO have published guidance on this:
Nucleic acid amplification testing is recommended for confirmation of the diagnosis.
The main confirmatory test is a positive reverse transcriptase-polymerase chain reaction (RT-PCR) for an Orthoebolavirus.[112]World Health Organization. Diagnostic testing for Ebola and Marburg virus diseases: interim guidance, 20 December 2024. Jan 2025 [internet publication].
https://www.who.int/publications/i/item/B09221
This test should be ordered in all patients with suspected infection while the patient is in isolation. It has the advantage of returning a result 24 to 48 hours before enzyme-linked immunosorbent assay (ELISA) testing. Several different commercial PCR kits are available with varying sensitivity, specificity, and limits of detection.[113]Cherpillod P, Schibler M, Vieille G, et al. Ebola virus disease diagnosis by real-time RT-PCR: a comparative study of 11 different procedures. J Clin Virol. 2016 Apr;77:9-14.
https://www.journalofclinicalvirology.com/article/S1386-6532(16)00040-8/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/26874083?tool=bestpractice.com
In Western settings, the test may only be available in regional or national laboratories that have category 4 facilities.[7]Feldmann H, Geisbert TW. Ebola haemorrhagic fever. Lancet. 2011 Mar 5;377(9768):849-62.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3406178
http://www.ncbi.nlm.nih.gov/pubmed/21084112?tool=bestpractice.com
In epidemic settings and some countries, category 4 laboratories are set up locally and results are available 4 hours after the sample has arrived.
Viral RNA can be detected in the patient’s blood by RT-PCR from day 3 up to days 6 to 17 of symptom onset. A positive PCR result implies that the patient is potentially infective, particularly if he or she has active diarrhea, vomiting, or bleeding. If the test is negative and the blood was drawn less than 72 hours after symptom onset, the test should be repeated with blood drawn more than 72 hours after symptom onset since viral load is low and can be undetectable early in the course of the illness. Negative tests should be repeated to rule out a diagnosis if it is strongly suspected (or confirm resolution of infection).[112]World Health Organization. Diagnostic testing for Ebola and Marburg virus diseases: interim guidance, 20 December 2024. Jan 2025 [internet publication].
https://www.who.int/publications/i/item/B09221
Higher viral load correlates with adverse outcome and increased mortality.[20]Bah EI, Lamah MC, Fletcher T, et al. Clinical presentation of patients with Ebola virus disease in Conakry, Guinea. N Engl J Med. 2015 Jan 1;372(1):40-7.
https://www.nejm.org/doi/full/10.1056/NEJMoa1411249#t=article
http://www.ncbi.nlm.nih.gov/pubmed/25372658?tool=bestpractice.com
[21]Chertow DS, Kleine C, Edwards JK, et al. Ebola virus disease in West Africa - clinical manifestations and management. N Engl J Med. 2014 Nov 27;371(22):2054-7.
https://www.nejm.org/doi/full/10.1056/NEJMp1413084
http://www.ncbi.nlm.nih.gov/pubmed/25372854?tool=bestpractice.com
[22]Schieffelin JS, Shaffer JG, Goba A, et al; KGH Lassa Fever Program; Viral Hemorrhagic Fever Consortium; WHO Clinical Response Team. Clinical illness and outcomes in patients with Ebola in Sierra Leone. N Engl J Med. 2014 Nov 27;371(22):2092-100.
https://www.nejm.org/doi/full/10.1056/NEJMoa1411680#t=article
http://www.ncbi.nlm.nih.gov/pubmed/25353969?tool=bestpractice.com
[71]Sanchez A, Lukwiya M, Bausch D, et al. Analysis of human peripheral blood samples from fatal and nonfatal cases of Ebola (Sudan) hemorrhagic fever: cellular responses, virus load, and nitric oxide levels. J Virol. 2004 Oct;78(19):10370-7.
https://jvi.asm.org/content/78/19/10370.full
http://www.ncbi.nlm.nih.gov/pubmed/15367603?tool=bestpractice.com
[96]Hunt L, Gupta-Wright A, Simms V, et al. Clinical presentation, biochemical, and haematological parameters and their association with outcome in patients with Ebola virus disease: an observational cohort study. Lancet Infect Dis. 2015 Nov;15(11):1292-9.
http://www.ncbi.nlm.nih.gov/pubmed/26271406?tool=bestpractice.com
[114]Towner JS, Rollin PE, Bausch DG, et al. Rapid diagnosis of Ebola hemorrhagic fever by reverse transcription-PCR in an outbreak setting and assessment of patient viral load as a predictor of outcome. J Virol. 2004 Apr;78(8):4330-41.
https://jvi.asm.org/content/78/8/4330.full
http://www.ncbi.nlm.nih.gov/pubmed/15047846?tool=bestpractice.com
The choice of whether to test for infection depends on the patient's history and their risk of infection according to the algorithm below.
[Figure caption and citation for the preceding image starts]: Diagnostic pathway for the workup of suspected Ebola diseaseProduced by the BMJ Evidence Centre [Citation ends].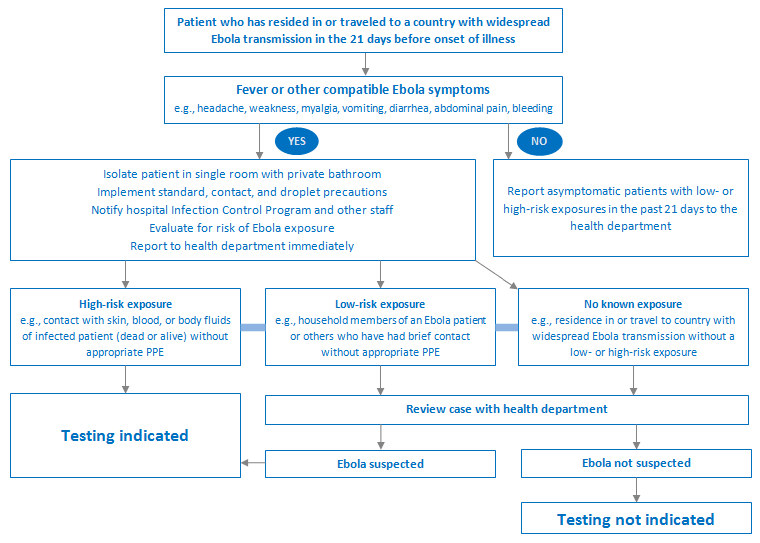
Testing is recommended for both the diagnosis of individuals who meet the case definition for a suspected case, and prior to discharging a confirmed case from a treatment center (two negative tests from blood samples taken at least 48 hours apart).[112]World Health Organization. Diagnostic testing for Ebola and Marburg virus diseases: interim guidance, 20 December 2024. Jan 2025 [internet publication].
https://www.who.int/publications/i/item/B09221
Malaria is still the most common cause of fever in people who live/work in, or travelers who have returned from, an endemic area and should be ruled out.[115]Boggild AK, Esposito DH, Kozarsky PE, et al. Differential diagnosis of illness in travelers arriving from Sierra Leone, Liberia, or Guinea: a cross-sectional study from the GeoSentinel Surveillance Network. Ann Intern Med. 2015 Jun 2;162(11):757-64.
http://www.ncbi.nlm.nih.gov/pubmed/25961811?tool=bestpractice.com
[116]Centers for Disease Control and Prevention. Guidance for malaria diagnosis in patients with suspected orthoebolavirus or orthomarburgvirus infection in the United States. Sep 2024 [internet publication].
https://www.cdc.gov/dpdx/malaria/malaria_ebola.html
Coinfection with malaria was seen in up to 5% of patients in West Africa during the 2014 outbreak, so the possibility of dual infection should be considered in all patients.[96]Hunt L, Gupta-Wright A, Simms V, et al. Clinical presentation, biochemical, and haematological parameters and their association with outcome in patients with Ebola virus disease: an observational cohort study. Lancet Infect Dis. 2015 Nov;15(11):1292-9.
http://www.ncbi.nlm.nih.gov/pubmed/26271406?tool=bestpractice.com
In the case of a positive rapid diagnostic test result for malaria, the infection should be treated while keeping in mind the patient's risk for Ebola disease and the possibility of a dual infection. Ebola disease should be considered in a patient who does not respond to antimalarial therapy.
It is recommended that appropriate confirmatory tests for Ebola disease are performed before, or in tandem with, differentiating tests for other suspected conditions if Ebola disease is suspected.
Other investigations
Traditionally, no other investigations outside of a malaria screen and RT-PCR were recommended due to the fear of putting laboratory workers at risk. However, it is now recognized that other investigations can be done safely according to recommended guidelines, as long as the laboratory is informed of the sample in advance, and the bloods are correctly packaged and retained at the end in case the RT-PCR is positive. Local protocols should be clear about safe transport of samples to the local and referral laboratories, and safe handling on receipt in the local laboratory.
The following investigations add valuable information to the workup and help guide further management, and should be ordered if possible. If investigations are limited due to the geographical location or facilities available, the most important tests to order are renal function, serum electrolytes, and blood lactate (if available).
Renal function and serum electrolytes:
Elevated serum creatinine or blood urea nitrogen and abnormal electrolytes may indicate acute kidney injury. This may be seen at the end of the first week of infection.[117]McElroy AK, Erickson BR, Flietstra TD, et al. Biomarker correlates of survival in pediatric patients with ebola virus disease. Emerg Infect Dis. 2014 Oct;20(10):1683-90.
https://wwwnc.cdc.gov/eid/article/20/10/14-0430_article
http://www.ncbi.nlm.nih.gov/pubmed/25279581?tool=bestpractice.com
Hypokalemia or hyperkalemia, due to vomiting and diarrhea or acute kidney injury, was seen in approximately 33% of cases in the 2014 outbreak.[96]Hunt L, Gupta-Wright A, Simms V, et al. Clinical presentation, biochemical, and haematological parameters and their association with outcome in patients with Ebola virus disease: an observational cohort study. Lancet Infect Dis. 2015 Nov;15(11):1292-9.
http://www.ncbi.nlm.nih.gov/pubmed/26271406?tool=bestpractice.com
Hypocalcemia has been associated with fatal infection. Hematuria and proteinuria may also be seen in severe disease. Oliguria that does not respond to fluid resuscitation is a poor prognostic sign.[16]Kortepeter MG, Bausch DG, Bray M. Basic clinical and laboratory features of filoviral hemorrhagic fever. J Infect Dis. 2011 Nov;204 Suppl 3:S810-6
https://jid.oxfordjournals.org/content/204/suppl_3/S810.long
http://www.ncbi.nlm.nih.gov/pubmed/21987756?tool=bestpractice.com
Blood lactate:
Elevated lactate is a marker of tissue hypoperfusion and is an indicator of shock. It is useful in acutely ill patients with signs of sepsis to identify the degree of systemic hypoperfusion and to guide fluid resuscitation.[118]Fowler RA, Fletcher T, Fischer WA 2nd, et al. Caring for critically ill patients with Ebola virus disease. Perspectives from West Africa. Am J Respir Crit Care Med. 2014 Oct 1;190(7):733-7.
https://www.atsjournals.org/doi/full/10.1164/rccm.201408-1514CP#.VEe1OvldWnA
http://www.ncbi.nlm.nih.gov/pubmed/25166884?tool=bestpractice.com
Elevated lactate was one indicator of gram-negative sepsis at day 15 in a patient treated in Germany.[46]Kreuels B, Wichmann D, Emmerich P, et al. A case of severe Ebola virus infection complicated by gram-negative septicemia. N Engl J Med. 2014 Dec 18;371(25):2394-401.
https://www.nejm.org/doi/full/10.1056/NEJMoa1411677#t=article
http://www.ncbi.nlm.nih.gov/pubmed/25337633?tool=bestpractice.com
Arterial blood gas:
Arterial or venous pH and bicarbonate are useful in acutely ill patients with signs of sepsis to identify the degree of systemic hypoperfusion and guide fluid resuscitation.[118]Fowler RA, Fletcher T, Fischer WA 2nd, et al. Caring for critically ill patients with Ebola virus disease. Perspectives from West Africa. Am J Respir Crit Care Med. 2014 Oct 1;190(7):733-7.
https://www.atsjournals.org/doi/full/10.1164/rccm.201408-1514CP#.VEe1OvldWnA
http://www.ncbi.nlm.nih.gov/pubmed/25166884?tool=bestpractice.com
Complete blood count:
Decreasing platelet count and marked lymphopenia can be seen in the initial stages of infection; however, this is not diagnostic. This is often followed by neutrophil leukocytosis in the later stages of patients who eventually recover, along with normalization of thrombocytopenia. Leukocytosis may persist and show immature forms. Patients with severe disease may show a progressive decline in platelet count as a manifestation of disseminated intravascular coagulation (DIC). Decreased hemoglobin levels were reported in 24% of patients in the 2014 outbreak,[96]Hunt L, Gupta-Wright A, Simms V, et al. Clinical presentation, biochemical, and haematological parameters and their association with outcome in patients with Ebola virus disease: an observational cohort study. Lancet Infect Dis. 2015 Nov;15(11):1292-9.
http://www.ncbi.nlm.nih.gov/pubmed/26271406?tool=bestpractice.com
and have been associated with bleeding in previous outbreaks.[16]Kortepeter MG, Bausch DG, Bray M. Basic clinical and laboratory features of filoviral hemorrhagic fever. J Infect Dis. 2011 Nov;204 Suppl 3:S810-6
https://jid.oxfordjournals.org/content/204/suppl_3/S810.long
http://www.ncbi.nlm.nih.gov/pubmed/21987756?tool=bestpractice.com
Coagulation studies:
Prolonged prothrombin time (PT) or activated partial thromboplastin time (aPTT) is associated with more severe infection and bleeding manifestations such as DIC. Also, patients with fatal infections have been found to have D-dimer levels four-fold higher on days 6 to 8 of infection compared with patients who survive.[119]Rollin PE, Bausch DG, Sanchez A. Blood chemistry measurements and D-Dimer levels associated with fatal and nonfatal outcomes in humans infected with Sudan Ebola virus. J Infect Dis. 2007 Nov 15;196 Suppl 2:S364-71.
https://jid.oxfordjournals.org/content/196/Supplement_2/S364.long
http://www.ncbi.nlm.nih.gov/pubmed/17940972?tool=bestpractice.com
Liver function tests:
Both alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are usually elevated; however, most studies show that AST rises out of proportion to ALT, and this is more suggestive of systemic tissue damage rather than hepatocellular injury.[96]Hunt L, Gupta-Wright A, Simms V, et al. Clinical presentation, biochemical, and haematological parameters and their association with outcome in patients with Ebola virus disease: an observational cohort study. Lancet Infect Dis. 2015 Nov;15(11):1292-9.
http://www.ncbi.nlm.nih.gov/pubmed/26271406?tool=bestpractice.com
The AST:ALT ratio peaked at 15:1 on days 6-8 of infection in fatal cases when compared with nonfatal cases, which had a peak of 5:1.[5]Formenty P, Hatz C, Le Guenno B, et al. Human infection due to Ebola virus, subtype Côte d'Ivoire: clinical and biologic presentation. J Infect Dis. 1999 Feb;179 Suppl 1:S48-53.
https://jid.oxfordjournals.org/content/179/Supplement_1/S48.long
http://www.ncbi.nlm.nih.gov/pubmed/9988164?tool=bestpractice.com
[16]Kortepeter MG, Bausch DG, Bray M. Basic clinical and laboratory features of filoviral hemorrhagic fever. J Infect Dis. 2011 Nov;204 Suppl 3:S810-6
https://jid.oxfordjournals.org/content/204/suppl_3/S810.long
http://www.ncbi.nlm.nih.gov/pubmed/21987756?tool=bestpractice.com
[119]Rollin PE, Bausch DG, Sanchez A. Blood chemistry measurements and D-Dimer levels associated with fatal and nonfatal outcomes in humans infected with Sudan Ebola virus. J Infect Dis. 2007 Nov 15;196 Suppl 2:S364-71.
https://jid.oxfordjournals.org/content/196/Supplement_2/S364.long
http://www.ncbi.nlm.nih.gov/pubmed/17940972?tool=bestpractice.com
Bilirubin, gamma glutamyltransferase, and alkaline phosphatase are often slightly elevated. Highly elevated ALT with severe jaundice suggests an alternative diagnosis (e.g., viral hepatitis).
Serum amylase:
Elevated levels have been reported in several studies and indicate the presence of pancreatitis, an indicator of severe infection.[16]Kortepeter MG, Bausch DG, Bray M. Basic clinical and laboratory features of filoviral hemorrhagic fever. J Infect Dis. 2011 Nov;204 Suppl 3:S810-6
https://jid.oxfordjournals.org/content/204/suppl_3/S810.long
http://www.ncbi.nlm.nih.gov/pubmed/21987756?tool=bestpractice.com
Serum blood glucose:
Hypoglycemia may be present in adults, but it is not commonly reported.[22]Schieffelin JS, Shaffer JG, Goba A, et al; KGH Lassa Fever Program; Viral Hemorrhagic Fever Consortium; WHO Clinical Response Team. Clinical illness and outcomes in patients with Ebola in Sierra Leone. N Engl J Med. 2014 Nov 27;371(22):2092-100.
https://www.nejm.org/doi/full/10.1056/NEJMoa1411680#t=article
http://www.ncbi.nlm.nih.gov/pubmed/25353969?tool=bestpractice.com
However, it is common in children and may be severe. It is a potentially reversible cause of confusion.[104]Fitzgerald F, Naveed A, Wing K, et al. Ebola virus disease in children, Sierra Leone, 2014-2015. Emerg Infect Dis. 2016 Oct;22(10):1769-77.
https://wwwnc.cdc.gov/eid/article/22/10/16-0579_article
http://www.ncbi.nlm.nih.gov/pubmed/27649367?tool=bestpractice.com
[105]Shah T, Greig J, van der Plas LM, et al. Inpatient signs and symptoms and factors associated with death in children aged 5 years and younger admitted to two Ebola management centres in Sierra Leone, 2014: a retrospective cohort study. Lancet Glob Health. 2016 Jul;4(7):e495-501.
https://www.thelancet.com/journals/langlo/article/PIIS2214-109X(16)30097-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/27340004?tool=bestpractice.com
Blood cultures:
Negative blood cultures are helpful as they rule out other nonviral infectious causes (e.g., sepsis, enteric fever). Gram-negative bacteremia, presumably from gut translocation, has been identified as a complication of the disease course in two patients.[46]Kreuels B, Wichmann D, Emmerich P, et al. A case of severe Ebola virus infection complicated by gram-negative septicemia. N Engl J Med. 2014 Dec 18;371(25):2394-401.
https://www.nejm.org/doi/full/10.1056/NEJMoa1411677#t=article
http://www.ncbi.nlm.nih.gov/pubmed/25337633?tool=bestpractice.com
[120]Dickson SJ, Clay KA, Adam M, et al. Enhanced case management can be delivered for patients with EVD in Africa: experience from a UK military Ebola treatment centre in Sierra Leone. J Infect. 2018 Apr;76(4):383-92.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5903873
http://www.ncbi.nlm.nih.gov/pubmed/29248587?tool=bestpractice.com
However, a study in Sierra Leone where blood cultures were taken from patients on admission to an Ebola treatment center found that only one of the 22 cultures was positive with a presumed contaminant.[121]Lamb L, Robson J, Ardley C, et al. Bacterial co-infection is rare in patients with Ebola virus disease in a military Ebola virus disease treatment unit in Sierra Leone. J Infect. 2015 Sep;71(3):406-7.
http://www.ncbi.nlm.nih.gov/pubmed/25818532?tool=bestpractice.com
Therefore, blood should be collected for culture at baseline and/or at the time of the onset of gastrointestinal symptoms or other clinical deterioration.
Antigen-capture enzyme-linked immunosorbent assay (ELISA) testing:
A useful diagnostic test with high specificity; however, it is not universally available. It is most likely to give a positive result from day 3-6 of infection, and can give widely variable results from days 7 to 16.[50]Rowe AK, Bertolli J, Khan AS, et al. Clinical, virologic, and immunologic follow-up of convalescent Ebola hemorrhagic fever patients and their household contacts, Kikwit, Democratic Republic of the Congo. Commission de Lutte contre les Epidémies à Kikwit. J Infect Dis. 1999 Feb;179 Suppl 1:S28-35.
http://www.ncbi.nlm.nih.gov/pubmed/9988162?tool=bestpractice.com
Can be used to confirm the diagnosis along with a positive RT-PCR result.
Serology:
Serologic tests that detect IgM and IgG antibodies are useful in the later stages of infection, particularly if other confirmatory tests yield inconclusive results, but should not be used alone for diagnosis.[112]World Health Organization. Diagnostic testing for Ebola and Marburg virus diseases: interim guidance, 20 December 2024. Jan 2025 [internet publication].
https://www.who.int/publications/i/item/B09221
IgM antibodies can appear in serum as early as day 2 post infection, but can give variable results up to day 9. They become negative between 30 and 168 days after symptom onset. An IgG response develops between day 6-18 and can persist for several years. A positive IgM or a rising IgG titer is strong evidence for recent infection.[50]Rowe AK, Bertolli J, Khan AS, et al. Clinical, virologic, and immunologic follow-up of convalescent Ebola hemorrhagic fever patients and their household contacts, Kikwit, Democratic Republic of the Congo. Commission de Lutte contre les Epidémies à Kikwit. J Infect Dis. 1999 Feb;179 Suppl 1:S28-35.
http://www.ncbi.nlm.nih.gov/pubmed/9988162?tool=bestpractice.com
Paired serum samples should be collected 21 days apart with the first sample being collected during the first week of illness.[112]World Health Organization. Diagnostic testing for Ebola and Marburg virus diseases: interim guidance, 20 December 2024. Jan 2025 [internet publication].
https://www.who.int/publications/i/item/B09221
Chest x-ray:
Useful in patients with respiratory symptoms. Pulmonary infiltrates are not typical of infection and suggest an alternative (or comorbid) diagnosis. May be difficult to arrange in an isolation unit and should only be ordered judiciously to avoid contamination.[122]Auffermann WF, Kraft CS, Vanairsdale S, et al. Radiographic imaging for patients with contagious infectious diseases: how to acquire chest radiographs of patients infected with the Ebola virus. AJR Am J Roentgenol. 2015 Jan;204(1):44-8.
http://www.ncbi.nlm.nih.gov/pubmed/25402496?tool=bestpractice.com
Rapid diagnostic tests
Rapid PCR testing remains a major hurdle for effective, targeted isolation of affected patients. Current tests take an average of 4 hours to perform with a fully equipped level 3 or 4 biosafety laboratory close at hand, but results may take several days to arrive in remote areas. This means that, until they are confirmed negative, patients with febrile illnesses other than Ebola disease are confined to isolation and often unwittingly exposed to the virus. Rapid bedside tests can therefore make a very significant contribution to infection control in treatment centers.
WHO: interim guidance on the use of rapid Ebola antigen detection tests
Opens in new window
Several different technologies are being evaluated by WHO for use in field conditions. These include numerous RT-PCR-based assays that have been made simpler to use with a shorter turnaround time of <1 hour. The WHO has listed ReEBOV™ Antigen Rapid Test Kit for potential use; however, it currently only recommends its use in special situations (e.g., postmortem diagnosis during an outbreak). The alternatives are ELISA-based antigen-detection assays that could be quicker and simpler with the possible advantage of only needing a drop of blood. Their major disadvantage is a reduced sensitivity, particularly in the initial stages of illness.[112]World Health Organization. Diagnostic testing for Ebola and Marburg virus diseases: interim guidance, 20 December 2024. Jan 2025 [internet publication].
https://www.who.int/publications/i/item/B09221
[123]Butler D. Ebola experts seek to expand testing. Nature. 2014 Dec 11;516(7530):154-5.
https://www.nature.com/news/ebola-experts-seek-to-expand-testing-1.16518
http://www.ncbi.nlm.nih.gov/pubmed/25503213?tool=bestpractice.com
[124]Burd EM. Ebola virus: a clear and present danger. J Clin Microbiol. 2015 Jan;53(1):4-8.
https://jcm.asm.org/content/53/1/4.long
http://www.ncbi.nlm.nih.gov/pubmed/25392362?tool=bestpractice.com
Nanopore technology may allow rapid detection and sequencing in the presence of very low levels of virus, and can potentially be deployed using a pocket-sized detection kit.[125]Hoenen T, Groseth A, Rosenke K, et al. Nanopore sequencing as a rapidly deployable ebola outbreak tool. Emerg Infect Dis. 2016 Feb;22(2):331-4.
https://wwwnc.cdc.gov/eid/article/22/2/15-1796_article
http://www.ncbi.nlm.nih.gov/pubmed/26812583?tool=bestpractice.com
[126]Tsang MK, Ye W, Wang G, et al. Ultrasensitive detection of ebola virus oligonucleotide based on upconversion nanoprobe/nanoporous membrane system. ACS Nano. 2016 Jan 26;10(1):598-605.
http://www.ncbi.nlm.nih.gov/pubmed/26720408?tool=bestpractice.com
Rapid sequencing of the virus using these new technologies during an outbreak could allow real-time understanding of viral dynamics.[127]Quick J, Loman NJ, Duraffour S, et al. Real-time, portable genome sequencing for Ebola surveillance. Nature. 2016 Feb 11;530(7589):228-32.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4817224
http://www.ncbi.nlm.nih.gov/pubmed/26840485?tool=bestpractice.com
A GeneXpert® diagnostic tool has been developed and trialed in the field. The Xpert® Ebola is an automated cartridge-based system that requires minimal laboratory skill. An inactivated sample is placed into a single-use cartridge, which is then entered into the enclosed machine. Sample preparation, nucleic acid amplification and detection, and production of a result are automated processes minimizing staff training requirements, risk of infection, and cross contamination.[128]Semper AE, Broadhurst MJ, Richards J, et al. Performance of the GeneXpert Ebola assay for diagnosis of Ebola virus disease in Sierra Leone: a field evaluation study. PLoS Med. 2016 Mar 29;13(3):e1001980.
https://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1001980
http://www.ncbi.nlm.nih.gov/pubmed/27023868?tool=bestpractice.com
Other test kits have also been granted emergency use authorization by the WHO.
This is an evolving field and different kits are approved according to the country and settings in which they are to be deployed.

