小结
定义
病史和体格检查
关键诊断因素
- 每日吸烟量
- 至吸第一支烟时间(TTFC) ≤30分钟或>30分钟
- 使用替代烟草和尼古丁递送产品
- 物质使用障碍(SUD)既往史
- 妊娠或哺乳
其他诊断因素
- 抑郁史
- 精神分裂症病史
- 癫痫
- 高血压
- 不稳定性心脏疾病
- 室性心律失常
- 哮喘
- 颞下颌关节或牙科疾病
危险因素
- 吸烟者
- 使用替代烟草和尼古丁递送产品
诊断性检查
首要检查
- 自报吸烟状况
- 可替宁水平
治疗流程
住院的主动吸烟者
准备戒烟的主动吸烟者:成人(不存在妊娠、母乳喂养、抑郁或物质使用障碍)
准备戒烟的主动吸烟者:成人(妊娠、哺乳、青少年)
主动吸烟者准备戒烟:抑郁和戒烟
准备戒烟的主动吸烟者:物质使用障碍和戒烟
主动吸烟者没有准备戒烟
无烟雾性烟草使用者
撰稿人
作者
Franck F. Rahaghi, MD, MHS, FCCP
Chairman
Pulmonary Department
Director
Pulmonary Education and Rehabilitation
Cleveland Clinic
Weston
FL
利益声明
FFR is a consultant and speaker for Bayer, United Therapeutics, Janssen-PH, Merck, Boehringer Ingleheim, Takeda, and Talecris. He is involved in industry-sponsored research with Bayer, Janssen-PH, Gossamer Bio, and Bellerophon.
Samuel Gurevich, MD, FCCP
Pulmonary Department
Director
Respiratory Therapy
Cleveland Clinic
Weston
FL
利益声明
SG declares that he has no competing interests.
鸣谢
Dr Franck F. Rahaghi and Dr Samuael Gurevich would like to gratefully acknowledge Dr Felix Hernandez, Dr Jose Gonzalez, and Dr Theodore W. Marcy, previous contributors to this topic.
利益声明
FH, JG, and TWM declare that they have no competing interests.
同行评议者
William Wadland, MD, MS
Professor and Chair
Department of Family Medicine
Associate Dean for Faculty Affairs and Development
College of Human Medicine
Michigan State University
East Lansing
MI
利益声明
WW declares that he has no competing interests.
Charles J. Bentz, MD, FACP
Medical Director
Tobacco Cessation and Prevention
Providence St. Vincent Hospital and Medical Center
Portland
OR
利益声明
CJB declares that he has no competing interests.
Fei-Ran Guo, MD
Department of Family Medicine
National Taiwan University Hospital
Taipei
Taiwan
利益声明
FRG received a grant from Pfizer, the manufacturer of varenicline, for one of the 3rd phase clinical trials of varenicline. FRG has also been reimbursed by Pfizer, Novartis (the manufacturer of nicotine patch), and GSK (the manufacturer of bupropion SR and nicotine lozenge), for attending conferences, and receiving fees for giving speeches on smoking cessation.
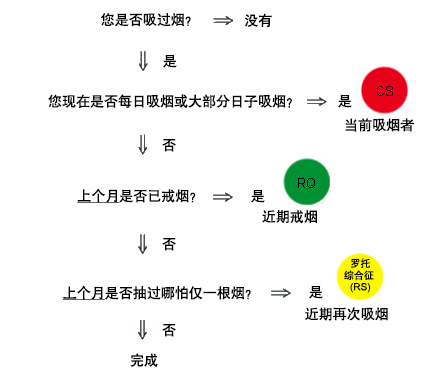
鉴别诊断
- 物质使用障碍
更多 鉴别诊断指南
- 烟草:预防吸食、促进戒烟以及治疗依赖
- 肿瘤学临床实践指南:戒烟
更多 指南患者教育信息
戒烟
更多 患者教育信息登录或订阅即可浏览 BMJ Best Practice 临床实践完整内容
内容使用需遵循免责声明