Resumo
Definição
História e exame físico
Principais fatores diagnósticos
- presença de fatores de risco
- massa mamária
- descarga mamilar
- linfadenopatia axilar
Outros fatores diagnósticos
- espessamento ou descoloração da pele
- retração, inversão ou descamação do mamilo
Fatores de risco
- idade mais avançada
- sexo feminino
- origem étnica
- história familiar positiva de câncer de mama e/ou outros tipos de câncer
- mutações genéticas em genes de suscetibilidade ao câncer de mama
- exposição ao estrogênio endógeno
- exposição ao estrogênio exógeno/progestina
- consumo de bebidas alcoólicas
- exposição à radiação
- doença atípica da mama
- aumento da densidade da mama
- realce parenquimatoso de fundo leve, moderado ou acentuado na ressonância nuclear magnética (RNM) da mama
- atividade física reduzida
- má alimentação
- condição socioeconômica alta
- tabagismo
- obesidade
- alta exposição ao dibutil-ftalato
Investigações diagnósticas
Primeiras investigações a serem solicitadas
- mamografia
Investigações a serem consideradas
- ultrassonografia das mamas
- RNM da mama
- biópsia
- perfil de receptores hormonais
- Teste de HER2
- ensaios da expressão gênica
- tomografia computadorizada
- teste genético
- Hemograma completo
- TFHs
- fosfatase alcalina
Algoritmo de tratamento
câncer de mama em estádio inicial (estádios I a IIB [T2 N1 M0])
câncer de mama avançado localmente (estádios IIB [T3 N0 M0] a III)
recorrência da doença
Colaboradores
Autores
Kandace P. McGuire, MD
Professor of Surgery
Virginia Commonwealth University
Richmond
VA
Declarações
KPM is on the Advisory Board for Kubtec and the speaker bureau for Endomag/Hologic.
Agradecimentos
Dr Kandace McGuire would like to gratefully acknowledge Dr Krystal Cascetta, Professor Amy Tiersten, Dr Hope S. Rugo, Dr Amal Melhem-Bertrandt, Dr Gabriel N. Hortobagyi, and Dr Phuong Khanh H. Morrow, the previous contributors to this topic.
Declarações
KC, AMB, GNH, and PKHM declare that they have no competing interests. AT is on the advisory board for Immunomedics, AstraZeneca, Novartis, Eisai, and Healthline; receives research funding from Pfizer, Novartis, Genentech, Lilly, and AstraZeneca; and does expert testimony work. HSR receives research funding through the University of California from Novartis, Pfizer, Genentech, Macrogenics, Plexxikon, Merck, Nektar, and GSK; has been reimbursed for travel by Novartis, Genentech, and Nektar; and has received speaker honorarium from Genomic Health.
Revisores
Savannah Liddell, MD
Fellow
Mayo Clinic College of Medicine and Science
Rochester
MN
Declarações
SL declares that she has no competing interests.
Katherine H.R. Tkaczuk, MD, FACP
Professor of Medicine
University of Maryland School of Medicine
Baltimore
MD
Declarações
KHRT declares that she has no competing interests.
Susan Tannenbaum, MD
Associate Professor, Medicine
Chief, Division of Hematology/Oncology
Medical Director, Neag Comprehensive Cancer Center
University of Connecticut
Farmington
CT
Declarações
ST declares that she has no competing interests.
Emily Hsu, MD
Fellow
Division of Hematology/Oncology
University of Connecticut
Farmington
CT
Declarações
EH declares that she has no competing interests.
Anees Chagpar, MD
Assistant Professor
University of Louisville
Louisville
KY
Declarações
AC declares that she has no competing interests.
Gurhan Celik, MD
General Surgeon
General Surgery Department
Istanbul Training and Research Hospital
Istanbul
Turkey
Declarações
GC is an author of a number of references cited in this topic.
Edward R. Sauter, MD, PhD
Medical Officer
Breast and Gynecologic Cancer Working Group
Division of Cancer Prevention
National Cancer Institute
Bethesda
MD
Declarações
ERS declares that he has no competing interests.
Créditos aos pareceristas
Os tópicos do BMJ Best Practice são constantemente atualizados, seguindo os desenvolvimentos das evidências e das diretrizes. Os pareceristas aqui listados revisaram o conteúdo pelo menos uma vez durante a história do tópico.
Declarações
As afiliações e declarações dos pareceristas referem--se ao momento da revisão.
Referências
Principais artigos
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis [internet publication].Texto completo
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer [internet publication].Texto completo
Loibl S, André F, Bachelot T, et al. Early breast cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2024 Feb;35(2):159-82.Texto completo Resumo
Denduluri N, Somerfield MR, Eisen A, et al. Selection of optimal adjuvant chemotherapy regimens for human epidermal growth factor receptor 2 (HER2)-negative and adjuvant targeted therapy for HER2-positive breast cancers: an American Society of Clinical Oncology guideline adaptation of the Cancer Care Ontario clinical practice guideline. J Clin Oncol. 2016 Jul 10;34(20):2416-27.Texto completo Resumo
Park KU, Somerfield MR, Anne N, et al. Sentinel lymph node biopsy in early-stage breast cancer: ASCO guideline update. J Clin Oncol. 2025 May 10;43(14):1720-41.Texto completo Resumo
Artigos de referência
Uma lista completa das fontes referenciadas neste tópico está disponível para os usuários com acesso total ao BMJ Best Practice.
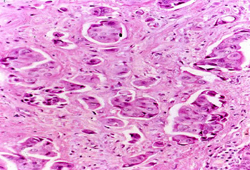
Diagnósticos diferenciais
- Alterações fibrocísticas
- Fibroadenoma
- Mastite
Mais Diagnósticos diferenciaisDiretrizes
- Suspected cancer: recognition and referral
- Sentinel lymph node biopsy for patients with early-stage breast cancer
Mais DiretrizesFolhetos informativos para os pacientes
Câncer de mama localmente avançado: o que é?
Câncer de mama: CDIS (câncer de mama em estágio muito inicial) em mulheres
Mais Folhetos informativos para os pacientesConectar-se ou assinar para acessar todo o BMJ Best Practice
O uso deste conteúdo está sujeito ao nosso aviso legal