Resumo
Definição
História e exame físico
Principais fatores diagnósticos
- inchaço da panturrilha
- dor localizada ao longo do sistema venoso profundo
Outros fatores diagnósticos
- Edema assimétrico
- veias superficiais proeminentes
- edema da perna inteira
- flegmasia cerulea dolens
Fatores de risco
- recentemente acamado por 3 dias ou mais
- cirurgia de grande porte nos 3 meses anteriores
- internação hospitalar nos 2 meses anteriores
- câncer ativo
- evento tromboembólico venoso prévio
- trauma ou fratura recente
- idade mais avançada
- gestação e período pós-parto
- veias varicosas
- paralisia dos membros inferiores
- trombofilias hereditárias
- fator V de Leiden
- mutação no gene G20210A da protrombina
- deficiência de proteína C ou proteína S
- deficiência de antitrombina
- síndrome antifosfolipídica
- comorbidade clínica
- uso de medicamentos específicos
- obesidade
- tabagismo
- viagem aérea recente de longa duração
- história familiar de tromboembolismo venoso
- cateterização venosa central
Investigações diagnósticas
Primeiras investigações a serem solicitadas
- escore de Wells
- nível quantitativo de dímero D
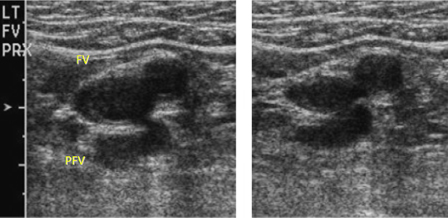
- ultrassonografia duplex (DUS) venosa
- razão normalizada internacional (INR) e tempo de tromboplastina parcial ativada (TTPa)
- ureia e creatinina
- TFHs
- Hemograma completo
Investigações a serem consideradas
- teste do fluxo venoso por Doppler
- tomografia computadorizada (TC) do abdome e da pelve com contraste
- rastreamento para trombofilia
Algoritmo de tratamento
suspeita ou confirmação de TVP do membro inferior com flegmasia cerulea dolens
suspeita ou confirmação de TVP sem flegmasia cerulea dolens e sem contraindicações à anticoagulação: terapia em fase inicial
suspeita ou confirmação de TVP sem flegmasia cerulea dolens: contraindicações à anticoagulação
fase de tratamento da terapia
terapia de fase prolongada: TVP com fatores precipitantes
terapia de fase prolongada: TVP sem fatores precipitantes
terapia de fase prolongada: gestantes
terapia de fase prolongada: associado a câncer
fase prolongada da terapia: tromboembolismo venoso recorrente
Colaboradores
Autores
Scott M. Stevens, MD
Director
Thrombosis Clinic
Intermountain Medical Center
Murray
Professor of Medicine
Department of Medicine
Intermountain Healthcare and University of Utah
Salt Lake City
UT
Declarações
SMS declares that he has no competing interests.
Scott C. Woller, MD
Director
Thrombosis Clinic
Intermountain Medical Center
Murray
Professor of Medicine
Department of Medicine
Intermountain Healthcare and University of Utah
Salt Lake City
UT
Declarações
SCW declares that he is expecting to receive funding of an investigator-initiated grant from Janssen Pharmaceuticals to Intermountain Health with no direct compensation to himself for research in the sum of $500,000 in 2024.
Gabriel V. Fontaine, PharmD, MBA, BCPS
Clinical Pharmacy Manager
Critical Care and Emergency Medicine
Advanced Clinical Pharmacist
Neuroscience Critical Care
Intermountain Medical Center
Murray
UT
Declarações
GVF has received consulting fees and honoraria from AstraZeneca, Chiesi, and Anticoagulation Forum.
Agradecimentos
Dr Scott M. Stevens, Dr Scott C. Woller, and Dr Gabriel V. Fontaine would like to gratefully acknowledge Dr Geno Merli, Dr Taki Galanis, Dr Luis Eraso, Dr Geoffrey Ouma, Dr Richard White, and Dr Windsor Ting, the previous contributors to this topic.
Declarações
GM has received grant or research support from BMS, J&J, Sanofi-Aventis, Portola, and Janssen; he has served as a Scientific Consultant for BMS, J&J, and Sanofi-Aventis. RW declares participation in numerous multicentred clinical trials sponsored by companies: Agenix, Boehringer-Ingleheim, Amgen, Bayer, Bristol-Meyer-Squibb, Novartis, Hemosense. TG, LE, GO, and WT declare that they have no competing interests.
Revisores
Beverly Hunt, FRCP, FRCPath, MD
Professor of Thrombosis & Haemostasis
King's College
Consultant
Departments of Haematology, Pathology & Rheumatology
Lead in Blood Sciences
Guy's & St Thomas' NHS Foundation Trust
London
UK
Declarações
BH declares that she has no competing interests.
Créditos aos pareceristas
Os tópicos do BMJ Best Practice são constantemente atualizados, seguindo os desenvolvimentos das evidências e das diretrizes. Os pareceristas aqui listados revisaram o conteúdo pelo menos uma vez durante a história do tópico.
Declarações
As afiliações e declarações dos pareceristas referem--se ao momento da revisão.
Referências
Principais artigos
Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021 Dec;160(6):e545-608.Texto completo Resumo
Mazzolai L, Ageno W, Alatri A, et al. Second consensus document on diagnosis and management of acute deep vein thrombosis: updated document elaborated by the ESC Working Group on aorta and peripheral vascular diseases and the ESC Working Group on pulmonary circulation and right ventricular function. Eur J Prev Cardiol. 2022 May 27;29(8):1248-63.Texto completo Resumo
Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012 Feb;141(2 suppl):e195S-226S.Texto completo Resumo
Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: compendium and review of CHEST guidelines 2012-2021. Chest. 2024 Aug;166(2):388-404.Texto completo Resumo
Artigos de referência
Uma lista completa das fontes referenciadas neste tópico está disponível para os usuários com acesso total ao BMJ Best Practice.
Diagnósticos diferenciais
- Celulite
- Laceração dos músculos da panturrilha/ruptura do tendão de Aquiles
- Hematoma nos músculos da panturrilha
Mais Diagnósticos diferenciaisDiretrizes
- NCCN clinical practice guidelines in oncology: cancer-associated venous thromboembolic disease
- ACR-AIUM-SPR-SRU practice parameter for the performance of peripheral venous ultrasound examination
Mais DiretrizesFolhetos informativos para os pacientes
Trombose venosa profunda e viagens de longa distância
Trombose venosa profunda
Mais Folhetos informativos para os pacientesCalculadoras
Probabilidade pré-teste de trombocitopenia induzida por heparina (escore de 4T)
Mais CalculadorasConectar-se ou assinar para acessar todo o BMJ Best Practice
O uso deste conteúdo está sujeito ao nosso aviso legal