Resumo
Definição
História e exame físico
Principais fatores diagnósticos
- presença de fatores de risco
- ingestão de leite, ovos, nozes, peixe, marisco, trigo ou soja
- sintomas reprodutíveis
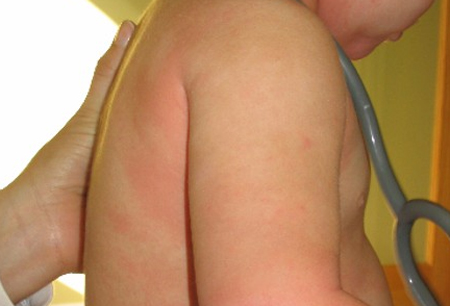
- rubor, urticária ou angioedema cutâneo
- espirros, rinorreia ou congestão nasal
- dispneia, taquipneia, sibilância, tosse ou cianose
- rouquidão, estridor ou sensação de sufocamento
- náuseas e vômitos
- cólica ou distensão abdominal
- diarreia
- hiperemia conjuntival ou lacrimejamento
- edema periorbital
- início abrupto dos sintomas
- reação causada por uma pequena quantidade de alimento
- presença de outras doenças alérgicas
- edema laríngeo
Outros fatores diagnósticos
- taquicardia ou bradicardia
- reação exacerbada por exercícios ou esforço físico
- ingestão de medicamentos ou de bebidas alcoólicas antes da reação
- arritmia cardíaca
- hipotensão
Fatores de risco
- história familiar de alergia alimentar
- dermatite atópica
- neonatos
- exposição perinatal a óleo de amendoim
Investigações diagnósticas
Primeiras investigações a serem solicitadas
- imunoensaio da imunoglobulina E (IgE) específica in vitro
- teste alérgico cutâneo por puntura ("prick test")
Investigações a serem consideradas
- testes de desencadeamento alimentar
- diagnósticos resolvidos por componentes
Novos exames
- testes de adesivo atópico
Algoritmo de tratamento
reação anafilática
sintomas cutâneos
broncoespasmo
rinoconjuntivite
após a estabilização
Colaboradores
Autores
A. Wesley Burks, MD

Curnen Distinguished Professor and Chair
Department of Pediatrics
University of North Carolina
Chapel Hill
NC
Declarações
AWB receives grant support to his institution from the National Institutes of Health and the Burroughs Wellcome Fund; royalties from UpToDate, Elsevier, and Walter Kluwer; consulting honorariums from Astella Pharma Global Development, Allergy Therapeutics (UK) Ltd, DBV Technologies, Kaléo, N-Fold, LLC, ALK-Abelló Inc, and UKKO Inc, as well as Aimmune Therapeutics, Consortia TX Inc, and Prota Therapeutics for his service on their respective scientific advisory boards. AWB owns stock in Allertein and Mastcell Pharmaceuticals. These interests do not directly relate to this topic but are being shared for full disclosure. AWB is an author of several references cited in this topic.
J. Andrew Bird, MD
Associate Professor
Department of Pediatrics
Division of Allergy and Immunology
University of Texas Southwestern Medical Center
Dallas
TX
Declarações
JAB consults for AllerGenis, Allergy Therapeutics Ltd, Before Brands, DBV Technologies, Genentech, and Novartis. He receives grant funding to his institution from Aimmune, DBV Technologies, Genentech, HIH-NIAD, Novartic, Siolta, and Regeneron. JAB is the author of one reference cited in this topic.
Revisores
Justin Skripak, MD
Assistant Professor of Pediatric Allergy and Immunology
Mount Sinai School of Medicine
New York
NY
Declarações
JS declares that he has no competing interests.
Hugh A. Sampson, MD
Professor of Pediatrics
Mount Sinai School of Medicine
New York
NY
Declarações
HAS holds a 4% interest in a biotech company, Allertein Pharmaceuticals LLC, which is developing an engineered recombinant protein vaccine for peanut allergy, and 45% interest in a virtual company, Herbal Springs LLC, that holds a patent application on a herbal product for treating asthma and another for treating food allergy. HAS is an author of several references cited in this topic.
Adam Fox, MA(Hons) Cantab., MSc, MBBS, DCH, FRCPCH, FHEA, Dip. Allergy
Consultant and Honorary Senior Lecturer in Paediatric Allergy
Evelina Children's Hospital
Guy's & St Thomas' Hospitals NHS Foundation Trust
London
UK
Declarações
AF declares that he has no competing interests.
Créditos aos pareceristas
Os tópicos do BMJ Best Practice são constantemente atualizados, seguindo os desenvolvimentos das evidências e das diretrizes. Os pareceristas aqui listados revisaram o conteúdo pelo menos uma vez durante a história do tópico.
Declarações
As afiliações e declarações dos pareceristas referem--se ao momento da revisão.
Referências
Principais artigos
Chafen JJ, Newberry SJ, Riedl MA, et al. Diagnosing and managing common food allergies: a systematic review. JAMA. 2010 May 12;303(18):1848-56. Resumo
Sicherer SH, Sampson HA. Food allergy: recent advances in pathophysiology and treatment. Annu Rev Med. 2009 Feb;60:261-77. Resumo
Greer FR, Sicherer SH, Burks AW, et al. The effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, hydrolyzed formulas, and timing of introduction of allergenic complementary foods. Pediatrics. 2019 Apr;143(4):e20190281.Texto completo Resumo
Muraro A, Halken S, Arshad SH, et al. EAACI food allergy and anaphylaxis guidelines: primary prevention of food allergy. Allergy. 2014 May;69(5):590-601.Texto completo Resumo
Sampson HA, Gerth van Wijk R, Bindslev-Jensen C, et al. Standardizing double-blind, placebo-controlled oral food challenges: American Academy of Allergy, Asthma & Immunology-European Academy of Allergy and Clinical Immunology PRACTALL consensus report. J Allergy Clin Immunol. 2012 Dec;130(6):1260-74.Texto completo Resumo
Heyman MB. American Academy of Pediatrics, Committee on Nutrition. Lactose intolerance in infants, children, and adolescents. Pediatrics. 2006 Sep;118(3):1279-86.Texto completo Resumo
Artigos de referência
Uma lista completa das fontes referenciadas neste tópico está disponível para os usuários com acesso total ao BMJ Best Practice.
Diagnósticos diferenciais
- Dermatite atópica
- Urticária
- Síndrome auriculotemporal
Mais Diagnósticos diferenciaisDiretrizes
- Diagnosis and management of celiac disease
- Anaphylaxis - a 2023 practice parameter
Mais DiretrizesFolhetos informativos para os pacientes
Alergia alimentar: o que é?
Alergia alimentar: que tratamentos estão disponíveis?
Mais Folhetos informativos para os pacientesConectar-se ou assinar para acessar todo o BMJ Best Practice
O uso deste conteúdo está sujeito ao nosso aviso legal