Резюме
Определение
Анамнез и осмотр
Ключевые диагностические факторы
- presença de fatores de risco
- ingestão de leite, ovos, nozes, peixe, marisco, trigo ou soja
- sintomas reprodutíveis
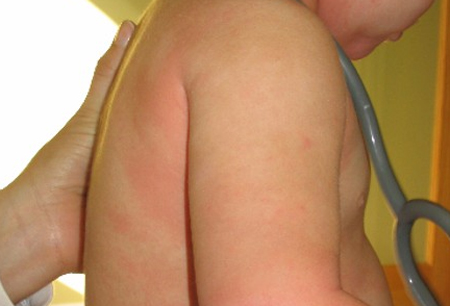
- rubor, urticária ou angioedema cutâneo
- espirros, rinorreia ou congestão nasal
- dispneia, taquipneia, sibilância, tosse ou cianose
- rouquidão, estridor ou sensação de sufocamento
- náuseas e vômitos
- cólica ou distensão abdominal
- diarreia
- hiperemia conjuntival ou lacrimejamento
- edema periorbital
- início abrupto dos sintomas
- reação causada por uma pequena quantidade de alimento
- presença de outras doenças alérgicas
- edema laríngeo
Другие диагностические факторы
- taquicardia ou bradicardia
- reação exacerbada por exercícios ou esforço físico
- ingestão de medicamentos ou de bebidas alcoólicas antes da reação
- arritmia cardíaca
- hipotensão
Факторы риска
- história familiar de alergia alimentar
- dermatite atópica
- neonatos
- exposição perinatal a óleo de amendoim
Диагностические исследования
Исследования, которые показаны в первую очередь
- imunoensaio da imunoglobulina E (IgE) específica in vitro
- teste alérgico cutâneo por puntura ("prick test")
Исследования, проведение которых нужно рассмотреть
- testes de desencadeamento alimentar
- diagnósticos resolvidos por componentes
Неотложные исследования
- testes de adesivo atópico
Алгоритм лечения
reação anafilática
sintomas cutâneos
broncoespasmo
rinoconjuntivite
após a estabilização
Составители
Авторы
A. Wesley Burks, MD

Curnen Distinguished Professor and Chair
Department of Pediatrics
University of North Carolina
Chapel Hill
NC
Раскрытие информации
AWB receives grant support to his institution from the National Institutes of Health and the Burroughs Wellcome Fund; royalties from UpToDate, Elsevier, and Walter Kluwer; consulting honorariums from Astella Pharma Global Development, Allergy Therapeutics (UK) Ltd, DBV Technologies, Kaléo, N-Fold, LLC, ALK-Abelló Inc, and UKKO Inc, as well as Aimmune Therapeutics, Consortia TX Inc, and Prota Therapeutics for his service on their respective scientific advisory boards. AWB owns stock in Allertein and Mastcell Pharmaceuticals. These interests do not directly relate to this topic but are being shared for full disclosure. AWB is an author of several references cited in this topic.
J. Andrew Bird, MD
Associate Professor
Department of Pediatrics
Division of Allergy and Immunology
University of Texas Southwestern Medical Center
Dallas
TX
Раскрытие информации
JAB consults for AllerGenis, Allergy Therapeutics Ltd, Before Brands, DBV Technologies, Genentech, and Novartis. He receives grant funding to his institution from Aimmune, DBV Technologies, Genentech, HIH-NIAD, Novartic, Siolta, and Regeneron. JAB is the author of one reference cited in this topic.
Рецензенты
Justin Skripak, MD
Assistant Professor of Pediatric Allergy and Immunology
Mount Sinai School of Medicine
New York
NY
Раскрытие информации
JS declares that he has no competing interests.
Hugh A. Sampson, MD
Professor of Pediatrics
Mount Sinai School of Medicine
New York
NY
Раскрытие информации
HAS holds a 4% interest in a biotech company, Allertein Pharmaceuticals LLC, which is developing an engineered recombinant protein vaccine for peanut allergy, and 45% interest in a virtual company, Herbal Springs LLC, that holds a patent application on a herbal product for treating asthma and another for treating food allergy. HAS is an author of several references cited in this topic.
Adam Fox, MA(Hons) Cantab., MSc, MBBS, DCH, FRCPCH, FHEA, Dip. Allergy
Consultant and Honorary Senior Lecturer in Paediatric Allergy
Evelina Children's Hospital
Guy's & St Thomas' Hospitals NHS Foundation Trust
London
UK
Раскрытие информации
AF declares that he has no competing interests.
Peer reviewer acknowledgements
BMJ Best Practice topics are updated on a rolling basis in line with developments in evidence and guidance. The peer reviewers listed here have reviewed the content at least once during the history of the topic.
Disclosures
Peer reviewer affiliations and disclosures pertain to the time of the review.
Список литературы
Основные статьи
Chafen JJ, Newberry SJ, Riedl MA, et al. Diagnosing and managing common food allergies: a systematic review. JAMA. 2010 May 12;303(18):1848-56. Аннотация
Sicherer SH, Sampson HA. Food allergy: recent advances in pathophysiology and treatment. Annu Rev Med. 2009 Feb;60:261-77. Аннотация
Greer FR, Sicherer SH, Burks AW, et al. The effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, hydrolyzed formulas, and timing of introduction of allergenic complementary foods. Pediatrics. 2019 Apr;143(4):e20190281.Полный текст Аннотация
Muraro A, Halken S, Arshad SH, et al. EAACI food allergy and anaphylaxis guidelines: primary prevention of food allergy. Allergy. 2014 May;69(5):590-601.Полный текст Аннотация
Sampson HA, Gerth van Wijk R, Bindslev-Jensen C, et al. Standardizing double-blind, placebo-controlled oral food challenges: American Academy of Allergy, Asthma & Immunology-European Academy of Allergy and Clinical Immunology PRACTALL consensus report. J Allergy Clin Immunol. 2012 Dec;130(6):1260-74.Полный текст Аннотация
Heyman MB. American Academy of Pediatrics, Committee on Nutrition. Lactose intolerance in infants, children, and adolescents. Pediatrics. 2006 Sep;118(3):1279-86.Полный текст Аннотация
Статьи, указанные как источники
A full list of sources referenced in this topic is available to users with access to all of BMJ Best Practice.
Отличия
- Dermatite atópica
- Urticária
- Síndrome auriculotemporal
Больше ОтличияРекомендации
- Diagnosis and management of celiac disease
- Anaphylaxis - a 2023 practice parameter
Больше РекомендацииЛифлеты для пациента
Alergia alimentar: o que é?
Alergia alimentar: que tratamentos estão disponíveis?
Больше Лифлеты для пациентаВойдите в учетную запись или оформите подписку, чтобы получить полноценный доступ к BMJ Best Practice
Использование этого контента попадает под действие нашего заявления об отказе от ответственности