Resumo
Definição
História e exame físico
Principais fatores diagnósticos
- presença de fatores de risco
- febre
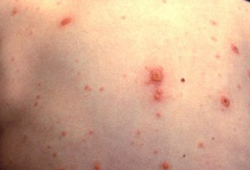
- erupção cutânea vesicular
- vesículas em membranas mucosas
Outros fatores diagnósticos
- prurido
- cefaleia
- fadiga/mal-estar
- faringite
- taquicardia
Fatores de risco
- exposição à varicela
- idade entre 1 e 9 anos
- estado não imunizado
- exposição ocupacional
Investigações diagnósticas
Primeiras investigações a serem solicitadas
- diagnóstico clínico
Investigações a serem consideradas
- reação em cadeia da polimerase
- cultura viral
- teste de anticorpo fluorescente direto (AFD)
- esfregaço de Tzanck
- aglutinação em látex (AL)
- ensaio de imunoadsorção enzimática (ELISA)
- fixação de complemento
- ultrassonografia (gestantes)
Algoritmo de tratamento
crianças saudáveis com baixo risco de contrair a forma mais grave da doença
aumento do risco de doença moderada a grave
alto risco de contrair a forma mais grave da doença
doença grave
Colaboradores
Autores
Andrew Riordan, MD, FRCPCH, MRCP, DTM&H
Consultant in Paediatric Infectious Diseases and Immunology
Royal Liverpool Children's Hospital (Alder Hey)
Liverpool
UK
Declarações
AR declares that he has no competing interests.
Agradecimentos
Dr Andrew Riordan would like to gratefully acknowledge Dr Steven Pergam, Dr Rupali Jain, and Dr Anna Wald, previous contributors to this topic.
Declarações
SP has served as a consultant for Merck & Co., Inc, Optimer/Cubist, and Chimerix, and has participated in clinical trials with these three organizations. He is also an author of a paper cited in this topic. RJ declares that she has no competing interests. AW is a member of the Data and Safety Monitoring Board (DSMB) for a Merck study of a candidate VZV vaccine.
Revisores
Chad M. Hivnor, Major, USAF, MC, FS
Chief
Outpatient & Pediatric Dermatology
59th Medical Wing/ SGOMD
Lackland Air Force Base
San Antonio
TX
Declarações
CMH declares that he has no competing interests.
Créditos aos pareceristas
Os tópicos do BMJ Best Practice são constantemente atualizados, seguindo os desenvolvimentos das evidências e das diretrizes. Os pareceristas aqui listados revisaram o conteúdo pelo menos uma vez durante a história do tópico.
Declarações
As afiliações e declarações dos pareceristas referem--se ao momento da revisão.
Referências
Principais artigos
UK Health Security Agency. The Green Book: Varicella. Jun 2019 [internet publication].Texto completo
Marin M, Güris D, Chaves SS, et al; Centers for Disease Control and Prevention. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007 Jun 22;56(RR-4):1-40.Texto completo Resumo
Royal College of Obstetricians and Gynaecologists. Chickenpox in pregnancy (Green-top guideline no. 13). January 2015 [internet publication].Texto completo
American Academy of Pediatrics. Varicella-zoster virus infections. In: Kimberlin DW, ed. Red book 2021- 2024: report of the Committee on Infectious Diseases. 32nd ed. Elk Grove Village, IL: American Academy of Pediatrics; 2021; 831-43.Texto completo
Pergam SA, Limaye AP, AST Infectious Diseases Community of Practice. Varicella zoster virus in solid organ transplantation: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019 Sep;33(9):e13622. Resumo
Centers for Disease Control and Prevention. Chickenpox (varicella). For healthcare professionals. Oct 2022 [internet publication].Texto completo
Centers for Disease Control and Prevention. Updated recommendations for use of VariZIG - United States, 2013. MMWR Morb Mortal Wkly Rep. 2013 Jul 19;62(28):574-6.Texto completo Resumo
Artigos de referência
Uma lista completa das fontes referenciadas neste tópico está disponível para os usuários com acesso total ao BMJ Best Practice.
Diagnósticos diferenciais
- Varíola
- Infecção por herpes-zóster
- Infecção por vírus do herpes simples (HSV)
Mais Diagnósticos diferenciaisDiretrizes
- Adult immunization schedule by age: recommendations for ages 19 years or older, United States, 2025
- Child and adolescent immunization schedule by age: recommendations for ages 18 years or younger, United States, 2025
Mais DiretrizesFolhetos informativos para os pacientes
Vacina contra herpes-zóster
Vacina contra varicela
Mais Folhetos informativos para os pacientesConectar-se ou assinar para acessar todo o BMJ Best Practice
Use of this content is subject to our disclaimer
