Resumo
Definição
História e exame físico
Principais fatores diagnósticos
- presença de fatores de risco
- história de sangramento recorrente ou intenso
- sangramento nos músculos
- sangramento prolongado após picada no calcanhar ou circuncisão
- sangramento mucocutâneo
- hemartrose
- pseudotumor
- sangramento intracraniano
Outros fatores diagnósticos
- contusões musculares/hematomas excessivos
- fadiga
- menorragia e sangramento após procedimentos cirúrgicos ou parto (mulheres portadoras)
- púrpura cutânea extensa (hemofilia adquirida)
- hemorragia digestiva e hematúria
- abdome distendido e doloroso
- palidez, taquicardia, taquipneia ou hipotensão
Fatores de risco
- história familiar de hemofilia (hemofilia congênita)
- sexo masculino (hemofilia congênita)
- >60 anos de idade (hemofilia adquirida)
- doenças autoimunes, doença inflamatória intestinal, diabetes, hepatite, gestação e período pós-parto, neoplasia maligna, gamopatia monoclonal e uso de determinados medicamentos (hemofilia adquirida)
Investigações diagnósticas
Primeiras investigações a serem solicitadas
- tempo de tromboplastina parcial ativada (TTPa)
- ensaio dos fatores VIII e IX do plasma
- estudo misto
- Hemograma completo
- tempo de protrombina (TP)
- ensaio do fator de von Willebrand do plasma
- ensaio dos fatores V e VII do plasma
- ensaio dos fatores XI e XII do plasma
- tempo de fechamento/tempo de sangramento e estudos de agregação plaquetária
- aminotransferases hepáticas séricas (aspartato aminotransferase [AST] e alanina aminotransferase [ALT])
- radiografias simples de locais ósseos específicos
- análise de mutação de fator VIII ou IX pré-natal por amniocentese ou biópsia de vilosidade coriônica (BVC)
Investigações a serem consideradas
- tomografia computadorizada (TC) de crânio ou pescoço
- RNM de crânio ou pescoço
- ultrassonografia abdominal ou TC abdominopélvica
- endoscopia digestiva alta ou colonoscopia
- análise de mutação de fator VIII ou IX no sangue
- teste de rastreamento de inibidor de fator VIII ou IX do plasma
- ensaio de inibidor de Bethesda/ensaio de inibidor de Bethesda modificado (em amostra de plasma)
Algoritmo de tratamento
sangramento com risco de vida/membro
congênito: sangramento em articulação ou músculo sem risco de vida
congênito: sangramento sem risco de vida no trato urinário
congênito: sangramento oral ou nasal sem risco de vida
adquirida
inibidores do fator VIII ou IX
sem inibidores VIII/IX: hemofilia grave
sem inibidores VIII/IX: hemofilia leve-moderada com sangramentos recorrentes em uma única articulação
Colaboradores
Autores
Man-Chiu Poon, MD, FRCP (C), FACP
Professor Emeritus
Departments of Medicine, Pediatrics and Oncology
Cumming School of Medicine
University of Calgary
Calgary
Canada
Declarações
M-CP has been an ad hoc speaker for Bayer, Novo Nordisk, and Pfizer; attended advisory board meetings of Bioverativ/Sanofi, CSL Behring, KVR Pharmaceuticals, Novo Nordisk, Octapharma, Pfizer, Roche, and Takeda; received grant funding from Bayer and CSL Behring; and undertaken contract research for Novo Nordisk.
Adrienne Lee, MD, FRCP (C)
Clinical Assistant Professor
Department of Medicine
Cumming School of Medicine
University of Calgary
Calgary
Canada
Declarações
AL declares that she has no competing interests.
Agradecimentos
Professor Poon and Dr Lee would like to gratefully acknowledge Dr Nigel S. Key, Dr Paul Giangrande, Dr Nidra I. Rodriguez, and Dr W. Keith Hoots, the previous contributors to this topic.
Declarações
NSK has undertaken paid consultancy for Baxter Biosciences, Novo Nordisk, CSL Behring, and Bayer. He has received grant funding from Baxter. PG has undertaken paid consultancy and/or received lecture fees from the following companies involved in haemophilia care: Bayer, CSL Behring, NovoNordisk, Pfizer/ BPL, Octapharma, Biogen Idec, and Biotest. NSK, NIR, and WKH are authors of reference(s) cited in this topic.
Revisores
Louis Aledort, MD
The Mary Weinfeld Professor of Clinical Research in Hemophilia
Mount Sinai School of Medicine
New York
NY
Declarações
LA declares that he has no competing interests.
Christoph Pechlaner, MD
Associate Professor of Medicine
Innsbruck Medical University
Innsbruck
Austria
Declarações
CP declares that he has no competing interests.
Créditos aos pareceristas
Os tópicos do BMJ Best Practice são constantemente atualizados, seguindo os desenvolvimentos das evidências e das diretrizes. Os pareceristas aqui listados revisaram o conteúdo pelo menos uma vez durante a história do tópico.
Declarações
As afiliações e declarações dos pareceristas referem--se ao momento da revisão.
Referências
Principais artigos
Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020 Aug;26 Suppl 6:1-158.Texto completo Resumo
Collins PW, Chalmers E, Hart DP, et al. Diagnosis and treatment of factor VIII and IX inhibitors in congenital haemophilia: 4th edition. Br J Haematol. 2013 Jan;160(2):153-70.Texto completo Resumo
National Hemophilia Foundation Medical and Scientific Advisory Council (MASAC). MASAC Document 280 - MASAC recommendations concerning products licensed for the treatment of hemophilia and selected disorders of the coagulation system. Aug 2023 [internet publication].Texto completo
National Hemophilia Foundation Medical and Scientific Advisory Council (MASAC). Guidelines for emergency department management of individuals with hemophilia and other bleeding disorders. Dec 2019 [internet publication].Texto completo
Tiede A, Collins P, Knoebl P, et al. International recommendations on the diagnosis and treatment of acquired hemophilia A. Haematologica. 2020 Jul;105(7):1791-801.Texto completo Resumo
Artigos de referência
Uma lista completa das fontes referenciadas neste tópico está disponível para os usuários com acesso total ao BMJ Best Practice.
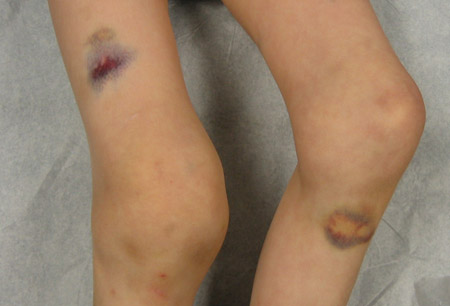
Diagnósticos diferenciais
- Doença de von Willebrand (DVW)
- Disfunção plaquetária
- Deficiência de outros fatores de coagulação (por exemplo, fator V, VII, X, XI ou fibrinogênio)
Mais Diagnósticos diferenciaisDiretrizes
- Recommendations regarding physical therapy management for the care of persons with bleeding disorders
- Recommendations concerning products licensed for the treatment of hemophilia and other bleeding disorders
Mais DiretrizesFolhetos informativos para os pacientes
Hepatite C: o que é?
Hepatite C: quais são as opções de tratamento?
Mais Folhetos informativos para os pacientesConectar-se ou assinar para acessar todo o BMJ Best Practice
O uso deste conteúdo está sujeito ao nosso aviso legal