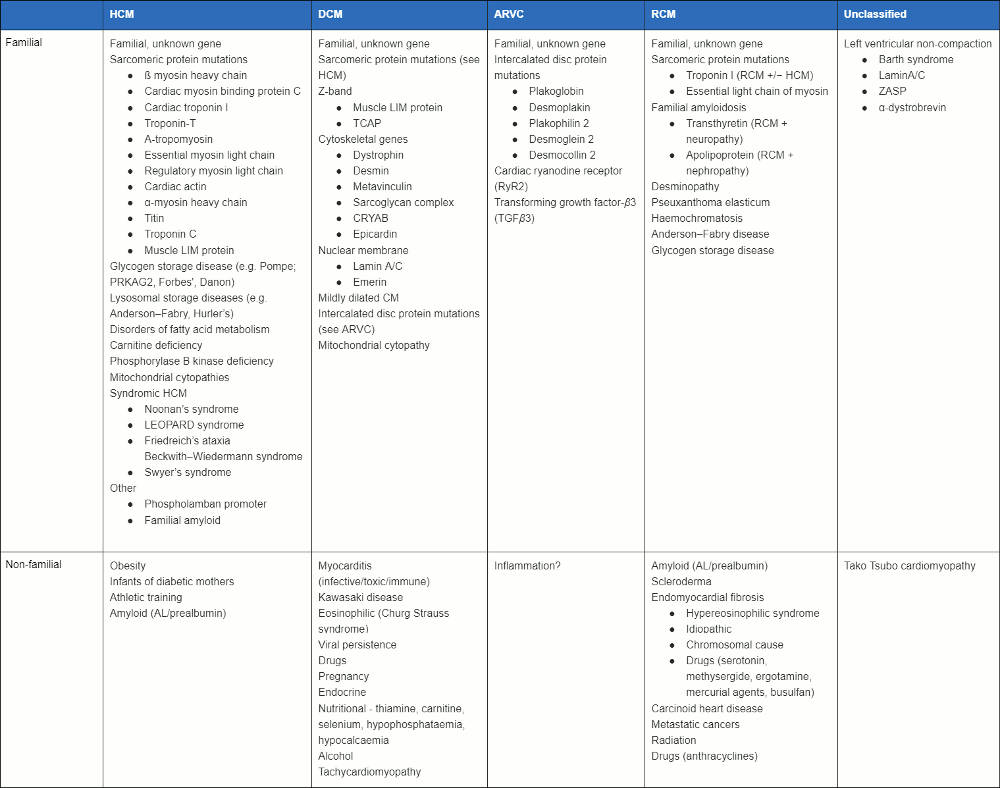
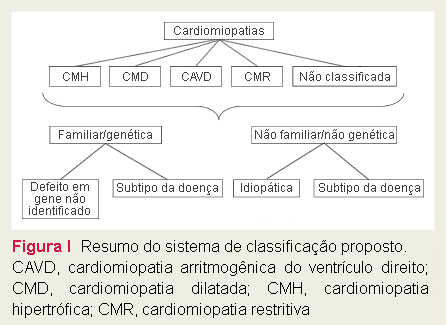
Summary
Differentials
Common
- Cardiomiopatia dilatada idiopática
- Miocardite
- Bebidas alcoólicas: cardiomiopatia dilatada
Uncommon
- Cardiomiopatia hipertrófica (CMH)
- Cardiomiopatia restritiva idiopática
- Cardiomiopatia arritmogênica do ventrículo direito
- Síndrome de Brugada e outras canalopatias iônicas
- Doença do sistema de condução
- Distúrbios mitocondriais
- Síndrome do takotsubo
- Periparto: cardiomiopatia dilatada
- Induzida por taquicardia: cardiomiopatia dilatada
- Amiloidose: cardiomiopatia hipertrófica ou restritiva
- Hemocromatose: cardiomiopatia restritiva ou dilatada
- Doença de Fabry: cardiomiopatia restritiva ou hipertrófica
- Outras doenças do armazenamento lisossomal
- Doxorrubicina: cardiomiopatia dilatada
- Metais pesados/substâncias químicas: cardiomiopatia dilatada
- Diabetes mellitus
- Disfunção tireoidiana: cardiomiopatia dilatada
- Acromegalia: cardiomiopatia hipertrófica ou dilatada
- Síndrome de Noonan: cardiomiopatia hipertrófica
- Lentiginose: cardiomiopatia hipertrófica
- Deficiência de tiamina (beribéri úmido): cardiomiopatia dilatada
- Ataxia de Friedreich/distrofia muscular: cardiomiopatia hipertrófica e dilatada
- Deficiência de ferro, niacina, selênio ou vitamina D: cardiomiopatia dilatada
- Lúpus eritematoso sistêmico: cardiomiopatia dilatada
- Fibrose endomiocárdica/endocardite de Loeffler (síndrome hipereosinofílica)
- Sarcoidose
- Distúrbios eletrolíticos
Contributors
Authors
Katie Linden, BMSc, MBChB
Cardiology Specialty Registrar
Belfast Health and Social Care Trust
Belfast
Northern Ireland
Disclosures
KL was reimbursed by Takeda UK Ltd., the manufacturer of Replagal for Fabry disease, for travel and accommodation for a leadership course. KL was reimbursed by Sanofi Aventis, the manufacturer of Genzyme for Fabry disease, for travel and accommodation for a conference. KL received a speaker fee from Sanofi Aventis, the manufacturer of Genzyme, for a talk on Fabry disease. There was no contractual right by Sanofi to control the content of this talk.
Alison Muir, MD
Consultant Cardiologist
Royal Victoria Hospital
Belfast Health and Social Care Trust
Belfast
UK
Disclosures
AM is an author of a reference cited in this topic.
Acknowledgements
KL and AM would like to gratefully acknowledge Dr Pascal McKeown, a previous contributor to this topic. PM is an author of a reference cited in this topic.
Peer reviewers
Lubna Choudhury, MB BCh, MD, MRCPI, FACC
Professor of Medicine
Division of Cardiology
Northwestern University
Chicago
IL
Disclosures
LC declares that she has no competing interests.
John Coltart, MD, FRCP, FACC, FESC, MRCS
Consultant Cardiologist
Cardio-thoracic Unit
Guy's and St Thomas' Hospital
London
UK
Раскрытие информации
JC declares that he has no competing interests.
Vaikom S. Mahadevan, MBBS, MRCP
Consultant Cardiologist
Manchester Royal Infirmary
Manchester
UK
Раскрытие информации
VSM declares that he has no competing interests.
Peer reviewer acknowledgements
BMJ Best Practice topics are updated on a rolling basis in line with developments in evidence and guidance. The peer reviewers listed here have reviewed the content at least once during the history of the topic.
Disclosures
Peer reviewer affiliations and disclosures pertain to the time of the review.
Список литературы
Основные статьи
Arbelo E, Protonotarios A, Gimeno JR, et al. 2023 ESC guidelines for the management of cardiomyopathies. Eur Heart J. 2023 Oct 1;44(37):3503-626.Полный текст Аннотация
Ommen SR, Ho CY, Asif IM, et al. 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR guideline for the management of hypertrophic cardiomyopathy: a report of the American Heart Association/American College of Cardiology joint committee on clinical practice guidelines. Circulation. 2024 Jun 4;149(23):e1239-311.Полный текст Аннотация
Nagueh SF, Phelan D, Abraham T, et al. Recommendations for multimodality cardiovascular imaging of patients with hypertrophic cardiomyopathy: an update from the American Society of Echocardiography, in collaboration with the American Society of Nuclear Cardiology, the Society for Cardiovascular Magnetic Resonance, and the Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr. 2022 Jun;35(6):533-69.Полный текст Аннотация
American College of Radiology. ACR appropriateness criteria: nonischemic myocardial disease with clinical manifestations. 2020 [internet publication].Полный текст
British Society of Echocardiography. Diagnosis and assessment of dilated cardiomyopathy: a guideline protocol from the British Society of Echocardiography. Jun 2017 [internet publication].Полный текст
Статьи, указанные как источники
A full list of sources referenced in this topic is available to users with access to all of BMJ Best Practice.

Рекомендации
- 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR guideline for the management of hypertrophic cardiomyopathy: a report of the American Heart Association/American College of Cardiology joint committee on clinical practice guidelines
- Management of cardiomyopathies
Больше РекомендацииЛифлеты для пациента
Insuficiência cardíaca: como posso me ajudar?
Insuficiência cardíaca
Больше Лифлеты для пациентаVideos
Galope de terceira bulha cardíaca
Galope de quarta bulha cardíaca
Больше ВидеоВойдите в учетную запись или оформите подписку, чтобы получить полноценный доступ к BMJ Best Practice
Использование этого контента попадает под действие нашего заявления об отказе от ответственности