Summary
Определение
Анамнез и осмотр
Ключевые диагностические факторы
- presença de fatores de risco
- história de exposição a medicamentos
- lesões cutâneas
- reações cutâneas variáveis em 5 a 15 minutos após a exposição ao medicamento
- reações cutâneas variáveis dentro de algumas horas após a exposição ao medicamento
- reações cutâneas variáveis dentro de 2 semanas após a exposição ao medicamento
- reações cutâneas variáveis dentro de meses a anos após a exposição ao medicamento
- exposição prévia e reação ao medicamento
Другие диагностические факторы
- prurido
- características não cutâneas associadas
- dor
Факторы риска
- infecções por vírus
- Infecção pelo vírus da imunodeficiência humana (HIV)
- polimorfismo HLA-B*5701
- polimorfismo HLA-B*1502
- polimorfismo HLA-B*5801
- sexo feminino
Диагностические исследования
Исследования, проведение которых нужно рассмотреть
- concentração de medicamentos no sangue (sangue total, plasma, soro)
- concentração de triptase no soro (anafilaxia)
- ensaio de via do complemento
- histologia da biópsia das lesões
- hemograma completo e diferencial
- anticorpos anti-histona para ácido desoxirribonucleico (DNA) de fita simples (síndrome semelhante a lúpus)
- testes cutâneos (testes de punção, intradérmicos, de contato)
Неотложные исследования
- imunoglobulina E (IgE) específica a medicamento
- teste de ativação de basófilos
- ensaio da proliferação linfocitária (EPL)
- ensaio de immunospot ligado a enzimas (teste ELISPOT)
Алгоритм лечения
reações cutâneas adversas graves
reações cutâneas adversas sem gravidade
após um episódio agudo
Составители
Авторы
Michael Ardern-Jones, BSc, MBBS, DPhil, FRCP
Associate Professor
Consultant Dermatologist
Faculty of Medicine
University of Southampton
Southampton
UK
Раскрытие информации
MA-J declares that he has no competing interests.
Выражение благодарностей
Dr Michael Ardern-Jones would like to gratefully acknowledge Dr Wei Yann Haw, Dr Anne Holbrook, Dr Hermenio Lima, and Dr Jeffrey K. Aronson, the previous contributors to this topic.
Раскрытие информации
WYH, AH and HL declare that they have no competing interests. JKA is editor of Meyler's Side Effects of Drugs and its annual companion volumes, the Side Effects of Drugs Annuals.
Рецензенты
Shahbaz A. Janjua, MD
Specialist Dermatologist
Ayza Skin & Research Center
Lalamusa
Pakistan
Divulgaciones
SAJ declares that he has no competing interests.
Craig K. Svensson, Pharm.D, PhD
Dean
College of Pharmacy, Nursing, and Health Sciences
Purdue University
West Lafayette
IN
Divulgaciones
CKS declares that he has no competing interests.
Agradecimiento de los revisores por pares
Los temas de BMJ Best Practice se actualizan de forma continua de acuerdo con los desarrollos en la evidencia y en las guías. Los revisores por pares listados aquí han revisado el contenido al menos una vez durante la historia del tema.
Divulgaciones
Las afiliaciones y divulgaciones de los revisores por pares se refieren al momento de la revisión.
Referencias
Artículos principales
Aronson JK, Ferner RE. Joining the DoTS: new approach to classifying adverse drug reactions. BMJ. 2003 Nov 22;327(7425):1222-5. Resumen
Gell PG, Coombs RRA, eds. Clinical aspects of immunology. 1st ed. Oxford: Blackwell; 1963.
Bigby M, Jick S, Jick H, et al. Drug-induced cutaneous reactions: a report from the Boston Collaborative Drug Surveillance Program on 15,438 consecutive inpatients, 1975 to 1982. JAMA. 1986 Dec 26;256(24):3358-63. Resumen
Kuokkanen K. Drug eruptions: a series of 464 cases in the Department of Dermatology, University of Turku, Finland, during 1966-1970. Acta Allergol. 1972 Dec;27(5):407-38. Resumen
Aronson JK, Ferner RE. Clarification of terminology in drug safety. Drug Saf. 2005;28(10):851-70. Resumen
Khan DA, Banerji A, Blumenthal KG, et al. Drug allergy: a 2022 practice parameter update. J Allergy Clin Immunol. 2022 Dec;150(6):1333-93.Texto completo
Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981 Aug;30(2):239-45. Resumen
Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965 Jan;58(1):295-300.Texto completo Resumen
Howick J, Glasziou P, Aronson JK. The evolution of evidence hierarchies: what can Bradford Hill's 'guidelines for causation' contribute? J R Soc Med. 2009 May;102(5):186-94.Texto completo Resumen
National Institute for Health and Care Excellence. Drug allergy: diagnosis and management. September 2014 [internet publication].Texto completo
Artículos de referencia
Una lista completa de las fuentes a las que se hace referencia en este tema está disponible para los usuarios con acceso a todo BMJ Best Practice.
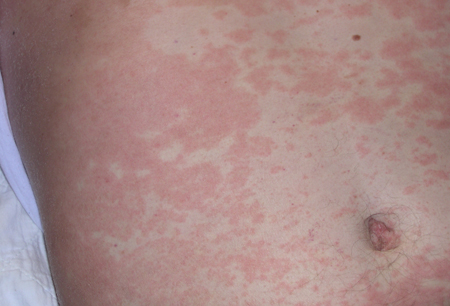
Diferenciales
- Lúpus eritematoso sistêmico
- Doenças bolhosas autoimunes
- Síndrome da pele escaldada estafilocócica
Más DiferencialesGuías de práctica clínica
- Anaphylaxis: a 2023 practice parameter update
- Drug allergy: a 2022 practice parameter
Más Guías de práctica clínicaInicie sesión o suscríbase para acceder a todo el BMJ Best Practice
El uso de este contenido está sujeto a nuestra cláusula de exención de responsabilidad