Resumo
Definição
Anamnesis y examen
Principales factores de diagnóstico
- presença de fatores de risco
- história familiar positiva
- presença de uma síndrome associada
- redução da visão periférica
- cegueira noturna
- comprometimento da adaptação ao escuro
- acuidade central reduzida
- atrofia do epitélio pigmentar da retina
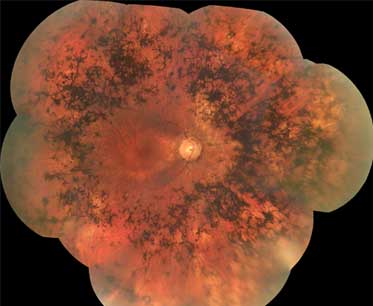
- pigmentação em espículas ósseas
Otros factores de diagnóstico
- nervo óptico pálido em cera
- fotopsias
- erro de refração
- catarata
- atenuação vascular retiniana
- edema macular cistoide
- células vítreas
- ofuscamento por luzes fortes
- visão das cores anormal
- ceratocone
- glaucoma
- drusas da cabeça do nervo óptico
- retinopatia tipo Coats
- amaurose congênita de Leber
Factores de riesgo
- história familiar
- presença de uma síndrome associada
Pruebas diagnósticas
Primeras pruebas diagnósticas para solicitar
- avaliação da acuidade visual
- exame do segmento anterior e medição da pressão intraocular
- perimetria total
- eletrorretinograma de campo total
Pruebas diagnósticas que deben considerarse
- limiar elevado final de adaptação ao escuro
- tomografia de coerência óptica (TCO)
- teste genético
- imagiologia óptica adaptativa
- autofluorescência de campo amplo de fundo do olho
Pruebas emergentes
- sequenciamento completo do exoma
Algoritmo de tratamiento
todos os pacientes
Colaboradores
Autores
Lesley Everett , MD, PhD, MPhil
Assistant Professor of Ophthalmology
Casey Eye Institute
Oregon Health and Sciences University
Divisions of Ophthalmic Genetics and Retina
Portland
OR
Divulgaciones
LAE is supported by a Foundation Fighting Blindness Career Development Grant.
Mark E. Pennesi, MD, PhD

Professor
Casey Eye Institute
Oregon Health and Sciences University
Portland
OR
Divulgaciones
MEP serves on the scientific advisory board and executive committee for the Foundation Fighting Blindness.
Paul Yang, MD, PhD

Associate Professor
Casey Eye Institute
Oregon Health and Sciences University
Portland
OR
Divulgaciones
PY acted as a consultant for 4D Molecular Therapeutics, AAVantgarde Bio (IDMC), Adverum, Astellas, Beacon Therapeutics, BlueRock Therapeutics, Eluminex Biosciences, Foundation Fighting Blindness (SAB), Janssen (DSMB), MieraGTx (DSMB), Nanoscope Therapeutics (SAB), and TeamedOn.
Agradecimientos
Dr Lesley Everett, Dr Mark E. Pennesi, and Dr Paul Yang would like to gratefully acknowledge Dr Richard G. Weleber and Dr Peter J. Francis, previous contributors to this topic.
Divulgaciones
RGW has served as a consultant to Novartis, Pfizer, and Wellstat, is a member of the scientific advisory board for Applied Genetic Technologies Corp, and serves on the scientific advisory board for the Foundation Fighting Blindness (the relationship has been reviewed and managed by Oregon Health & Science University). RGW also reports having received grants and personal fees from the Foundation Fighting Blindness and Applied Genetic Technologies Corp, and other support from Sanofi-Fovea, all outside the submitted work. In addition, RGW has a patent (US patent 8,657,446, Method and apparatus for visual field monitoring, also known as Visual Field Monitoring and Analysis, or VFMA, which has not been issued). PJF declares that he has no competing interests.
Revisores por pares
Scott Fraser, MD, FRCS (Ed), FRCOphth
Consultant Ophthalmologist
Sunderland Eye Infirmary
Sunderland
UK
Divulgaciones
SF declares that he has no competing interests.
Elias Traboulsi, MD
Professor of Ophthalmology
Director
Center for Genetic Eye Diseases
Cole Eye Institute
Cleveland Clinic
Cleveland
OH
Disclosures
ET declares that he has no competing interests.
Peer reviewer acknowledgements
BMJ Best Practice topics are updated on a rolling basis in line with developments in evidence and guidance. The peer reviewers listed here have reviewed the content at least once during the history of the topic.
Disclosures
Peer reviewer affiliations and disclosures pertain to the time of the review.
References
Key articles
American Academy of Ophthalmology. Comprehensive adult medical eye evaluation PPP. Nov 2020 [internet publication].Full text
American Academy of Ophthalmology. Guidelines on clinical assessment of patients with inherited retinal degenerations - 2022. Oct 2022 [internet publication].Full text
Robson AG, Frishman LJ, Grigg J, et al. ISCEV Standard for full-field clinical electroretinography (2022 update). Doc Ophthalmol. 2022 Jun;144(3):165-77.Full text Abstract
American Academy of Ophthalmology. Recommendations for genetic testing of inherited eye diseases. February 2014 [internet publication].Full text
Reference articles
A full list of sources referenced in this topic is available to users with access to all of BMJ Best Practice.
Differentials
- Rubéola congênita
- Sífilis
- Deficiência de vitamina A
More DifferentialsGuidelines
- Guidelines on clinical assessment of patients with inherited retinal degenerations
- Pediatric eye evaluations preferred practice pattern
More GuidelinesLog in or subscribe to access all of BMJ Best Practice
Use of this content is subject to our disclaimer