Resumo
Detalhes
- Técnicas de amostragem cervical
- Citologia cervical
- teste de vírus do papiloma humano (HPV)
- Vacinação contra o papilomavírus humano (HPV)
- Colposcopia
Colaboradores
Autores
Richard T. Penson, MD, MRCP

Medical Gynecologic Oncology
Division of Hematology Oncology
Massachusetts General Hospital
Boston
MA
Declarações
RTP reports personal fees for advisory boards from Aadi Bioscience, AstraZeneca, GSK Inc., ImmunoGen Inc., Merck & Co., Mersana, Novacure, Roche Pharma, Sutro Biopharma, and Vascular Biogenics Ltd; serves on Drug Safety and Monitoring Boards for AstraZeneca, EQRx, and Roche Pharma; Institutional research funding (as Principal Investigator) for Array BioPharma Inc., AstraZeneca., Eisai Inc., Genentech, Inc., Vascular Biogenics Ltd; and royalties from BMJ Publishing, UptoDate, Elsevier Ltd., Wolters Kluwer Health, and Wiley Blackwell. Payment for educational events: Research to Practice, ExpertConnect, ReachMD, CMEO Outfitters.
Marcela G. del Carmen, MD, MPH

President
Massachusetts General Physicians Organization
Division of Gynecologic-Oncology
Massachusetts General Hospital
Professor
Department of Obstetrics, Gynecology and Reproductive Biology
Harvard Medical School
Boston
MA
Declarações
MDC declares that she has no competing interests.
Revisores
Diane M. Harper, MD, MPH, MS
Professor
Departments of Family Medicine and Obstetrics and Gynecology
University of Michigan
Ann Arbor
MI
Declarações
DMH has been paid through a grant from Roche to the University of Michigan.
Deirdre Lyons, MB, BCh, BAO, MRCOG
Consultant in Obstetrics & Gynaecology
Lead Clinician in Colposcopy
Imperial College Healthcare NHS Trust
London
UK
Declarações
DL declares that she has no competing interests.
Matejka Rebolj, PhD
Scientific Researcher
Centre for Epidemiology and Screening
Institute of Public Health
University of Copenhagen
Copenhagen
Denmark
Declarações
MR declares that she has no competing interests.
Créditos aos pareceristas
Os tópicos do BMJ Best Practice são constantemente atualizados, seguindo os desenvolvimentos das evidências e das diretrizes. Os pareceristas aqui listados revisaram o conteúdo pelo menos uma vez durante a história do tópico.
Declarações
As afiliações e declarações dos pareceristas referem--se ao momento da revisão.
Referências
Principais artigos
Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020 Sep;70(5):321-46.Texto completo Resumo
Sawaya GF, Kulasingam S, Denberg TD, et al. Cervical cancer screening in average-risk women: best practice advice from the clinical guidelines committee of the American College of Physicians. Ann Intern Med. 2015;162:851-9.Texto completo Resumo
US Preventive Services Task Force, Curry SJ, Krist AH, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018 Aug 21;320(7):674-86.Texto completo Resumo
Artigos de referência
Uma lista completa das fontes referenciadas neste tópico está disponível para os usuários com acesso total ao BMJ Best Practice.
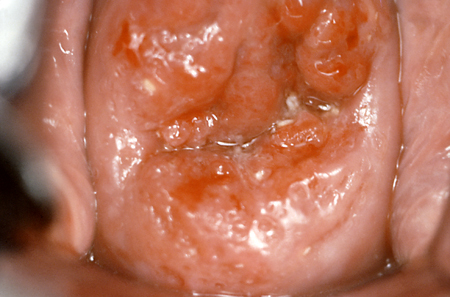
Diretrizes
- Updated cervical cancer screening guidelines
- Screening and treatment of cervical pre-cancer lesions for cervical cancer prevention
Mais DiretrizesConectar-se ou assinar para acessar todo o BMJ Best Practice
O uso deste conteúdo está sujeito ao nosso aviso legal