Resumo
Definition
History and exam
Key diagnostic factors
- presença de fatores de risco
- disfagia
- odinofagia
- perda de peso
Other diagnostic factors
- soluços
- tosse pós-prandial/paroxística
Risk factors
- sexo masculino
- idade avançada
- tabagismo
- uso excessivo de álcool (carcinoma de células escamosas)
- Esôfago de Barrett (adenocarcinoma)
- DRGE (adenocarcinoma)
- hérnia de hiato (adenocarcinoma)
- história familiar de câncer esofágico ou outro tipo de câncer (carcinoma de células escamosas)
- condição socioeconômica baixa
- raça não branca (carcinoma de células escamosas)
- bebidas e alimentos em alta temperatura (carcinoma de células escamosas)
- consumo de chimarrão (carcinoma de células escamosas)
- baixa ingestão de frutas e verduras frescas
- síndromes de cânceres hereditários
- obesidade (adenocarcinoma)
- papilomavírus humano (carcinoma de células escamosas)
- acalasia
- deficiências de vitaminas e minerais (carcinoma de células escamosas)
- higiene bucal insatisfatória (carcinoma de células escamosas)
Diagnostic tests
1st tests to order
- endoscopia digestiva alta (EDA) com biópsia
- ultrassonografia endoscópica (USE) ± aspiração com agulha fina (AAF)
- tomografia computadorizada (TC) de tórax e abdominal
- tomografia por emissão de pósitrons com 2-flúor-2-desoxi-D-glucose (FDG-PET)
- testes moleculares e patológicos
Tests to consider
- perfil metabólico abrangente
- ressonância nuclear magnética (RNM) de tórax e abdome
- broncoscopia ± AAF
- toracoscopia e laparoscopia
- biópsia líquida
- testes de função pulmonar
- prova de esforço
- ecocardiograma
Treatment algorithm
doença limitada (cT1, cN0, M0)
doença localizada (cT2, cN0, M0): elegível para cirurgia
doença localizada (cT2, cN0, M0): não elegível para cirurgia
doença localmente avançada (cT3-4, cN1-3, M0): elegível para cirurgia
doença localmente avançada (cT3-4, cN1-3, M0): não elegível para cirurgia
doença metastática (M1)
doença recorrente
კონტრიბუტორები
ავტორები
Ravi Rajaram, MD MSc FACS
Assistant Professor
Thoracic and Cardiovascular Surgery
University of Texas MD Anderson Cancer Center
Houston
TX
გაფრთხილება:
RR is a research consultant for Johnson & Johnson and an educational proctor for Intuitive Surgical.
მადლიერება
Dr Ravi Rajaram would like to gratefully acknowledge Dr Naureen Starling, Dr Caroline Fong, Dr Mark J. Krasna, and Dr Ghulam Abbas, the previous contributors to this topic.
გაფრთხილება:
MJK is an author of several references cited in this topic. GA declares that he has no competing interests. NS has received research funding from AstraZeneca, BMS, and Pfizer; travel and accommodation funding from AstraZeneca, BMS, Eli Lilly, Merck, Roche, and MSD Oncology; honoraria from Eli Lilly, Merck Serono, MSD Oncology, Pierre Fabre, Servier, GSK, and Amgen. She has been on the advisory board for Pfizer, AstraZeneca, Servier, and MSD (Merck). NS is an Honorary Clinical Senior Lecturer within the Division of Clinical Studies at the Institute of Cancer Research and serves on the UK National Cancer Research Institute (NCRI) oesophagogastric sub-group. NS has acted as a clinical expert in oesophagogastric cancer for NICE (guideline committee and technology appraisal) and is an upper GI expert for International Cancer Benchmarking Partnership. She is a Trustee for Pancreatic Cancer UK and a member of the EORTC General Assembly representing The Royal Marsden, as well as a member of the European Society for Medical Oncology (ESMO) Gastrointestinal Faculty. Educational roles include the NIHR Training Lead for NIHR Biomedical Research Centre, member of the Cancer Research Centre of Excellence training committee, Deputy Training Program Director (one of three) for South London Medical Oncology Training, member of the pan-London specialist Medical oncology training committee, and member of the Medical Oncology National Recruitment steering committee. CF declares that she has received honoraria from Bristol Myers Squibb.
რეცენზენტები
Peter McCulloch, MBChB, MA, MD, FRCS (Ed), FRCS (Glas)
Clinical Reader in Surgery
Nuffield Department of Surgery
University of Oxford
Oxford
UK
გაფრთხილება:
PM declares that he has no competing interests.
Nikhil I. Khushalani, MD
Assistant Professor of Oncology
Roswell Park Cancer Institute
Buffalo
NY
Disclosures
NIK has received funding for the conduction of clinical trials and associated translational studies from Merck, Pfizer, and Astra-Zeneca. NIK has a grant from the National Comprehensive Cancer Network (from research support by Roche).
Peer reviewer acknowledgements
BMJ Best Practice topics are updated on a rolling basis in line with developments in evidence and guidance. The peer reviewers listed here have reviewed the content at least once during the history of the topic.
Disclosures
Peer reviewer affiliations and disclosures pertain to the time of the review.
References
Key articles
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: esophageal and esophagogastric junction cancers [internet publication].Full text
Obermannová R, Alsina M, Cervantes A, et al; ESMO Guidelines Committee. Oesophageal cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022 Oct;33(10):992-1004.Full text Abstract
van Hagen P, Hulshof MC, van Lanschot JJ, et al; CROSS Group. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012 May 31;366(22):2074-84.Full text Abstract
Shapiro J, van Lanschot JJ, Hulshof MC, et al; CROSS study group. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015 Sep;16(9):1090-8. Abstract
Reference articles
A full list of sources referenced in this topic is available to users with access to all of BMJ Best Practice.
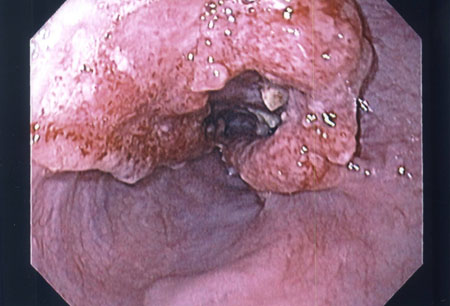
Differentials
- Estenose benigna
- Acalasia
- esôfago de Barrett
More DifferentialsGuidelines
- NCCN clinical practice guidelines in oncology: esophageal and esophagogastric junction cancers
- NCCN clinical practice guidelines in oncology: management of immunotherapy-related toxicities
More GuidelinesPatient information
Abandono do hábito de fumar
Câncer esofágico
More Patient informationLog in or subscribe to access all of BMJ Best Practice
Use of this content is subject to our disclaimer