შეჯამება
Definição
História e exame físico
Principais fatores diagnósticos
- presença de fatores de risco
- disfagia
- odinofagia
- perda de peso
Outros fatores diagnósticos
- soluços
- tosse pós-prandial/paroxística
Fatores de risco
- sexo masculino
- idade avançada
- tabagismo
- uso excessivo de álcool (carcinoma de células escamosas)
- Esôfago de Barrett (adenocarcinoma)
- DRGE (adenocarcinoma)
- hérnia de hiato (adenocarcinoma)
- história familiar de câncer esofágico ou outro tipo de câncer (carcinoma de células escamosas)
- condição socioeconômica baixa
- raça não branca (carcinoma de células escamosas)
- bebidas e alimentos em alta temperatura (carcinoma de células escamosas)
- consumo de chimarrão (carcinoma de células escamosas)
- baixa ingestão de frutas e verduras frescas
- síndromes de cânceres hereditários
- obesidade (adenocarcinoma)
- fatores alimentares (adenocarcinoma)
- papilomavírus humano (carcinoma de células escamosas)
- acalasia
- deficiências de vitaminas e minerais (carcinoma de células escamosas)
- higiene bucal insatisfatória (carcinoma de células escamosas)
Investigações diagnósticas
Primeiras investigações a serem solicitadas
- endoscopia digestiva alta (EDA) com biópsia
- ultrassonografia endoscópica (USE) ± aspiração com agulha fina (AAF)
- tomografia computadorizada (TC) de tórax e abdominal
- tomografia por emissão de pósitrons com 2-flúor-2-desoxi-D-glucose (FDG-PET)
- testes moleculares e patológicos
Investigações a serem consideradas
- perfil metabólico abrangente
- ressecção endoscópica
- ressonância nuclear magnética (RNM) de tórax e abdome
- broncoscopia ± AAF
- toracoscopia e laparoscopia
- DNA do tumor circulante
- testes de função pulmonar
- prova de esforço
- ecocardiograma
Algoritmo de tratamento
doença limitada (cT1, cN0, M0)
doença localizada (cT2, cN0, M0): elegível para cirurgia
doença localizada (cT2, cN0, M0): não elegível para cirurgia
doença localmente avançada (cT3-4, cN1-3, M0): elegível para cirurgia
doença localmente avançada (cT3-4, cN1-3, M0): não elegível para cirurgia
doença metastática (M1)
doença recorrente
Colaboradores
Autores
Ravi Rajaram, MD MSc FACS
Associate Professor
Thoracic and Cardiovascular Surgery
University of Texas MD Anderson Cancer Center
Houston
TX
Declarações
RR is a research consultant for Johnson & Johnson and an educational proctor for Intuitive Surgical.
Jenny Jing Li, MD
Assistant Professor
Gastrointestinal Medical Oncology
University of Texas MD Anderson Cancer Center
Houston
TX
利益声明
JJL has participated in advisory boards for BeOne Medicines and Gilead Sciences and conducted an educational workshop for AstraZeneca.
鸣谢
Dr Ravi Rajaram and Dr Jenny Jing Li would like to gratefully acknowledge Dr Naureen Starling, Dr Caroline Fong, Dr Mark J. Krasna, and Dr Ghulam Abbas, the previous contributors to this topic.
利益声明
MJK is an author of several references cited in this topic. GA declares that he has no competing interests. NS has received research funding from AstraZeneca, BMS, and Pfizer; travel and accommodation funding from AstraZeneca, BMS, Eli Lilly, Merck, Roche, and MSD Oncology; honoraria from Eli Lilly, Merck Serono, MSD Oncology, Pierre Fabre, Servier, GSK, and Amgen. She has been on the advisory board for Pfizer, AstraZeneca, Servier, and MSD (Merck). NS is an Honorary Clinical Senior Lecturer within the Division of Clinical Studies at the Institute of Cancer Research and serves on the UK National Cancer Research Institute (NCRI) oesophagogastric sub-group. NS has acted as a clinical expert in oesophagogastric cancer for NICE (guideline committee and technology appraisal) and is an upper GI expert for International Cancer Benchmarking Partnership. She is a Trustee for Pancreatic Cancer UK and a member of the EORTC General Assembly representing The Royal Marsden, as well as a member of the European Society for Medical Oncology (ESMO) Gastrointestinal Faculty. Educational roles include the NIHR Training Lead for NIHR Biomedical Research Centre, member of the Cancer Research Centre of Excellence training committee, Deputy Training Program Director (one of three) for South London Medical Oncology Training, member of the pan-London specialist Medical oncology training committee, and member of the Medical Oncology National Recruitment steering committee. CF declares that she has received honoraria from Bristol Myers Squibb.
同行评议者
Satish Maharaj, MD
Assistant Professor
Moffitt Cancer Center
University of South Florida
Tampa
FL
利益声明
SM declares that he has no competing interests.
Peter McCulloch, MBChB, MA, MD, FRCS (Ed), FRCS (Glas)
Clinical Reader in Surgery
Nuffield Department of Surgery
University of Oxford
Oxford
UK
利益声明
PM declares that he has no competing interests.
Nikhil I. Khushalani, MD
Assistant Professor of Oncology
Roswell Park Cancer Institute
Buffalo
NY
利益声明
NIK has received funding for the conduction of clinical trials and associated translational studies from Merck, Pfizer, and Astra-Zeneca. NIK has a grant from the National Comprehensive Cancer Network (from research support by Roche).
Peer reviewer acknowledgements
BMJ Best Practice topics are updated on a rolling basis in line with developments in evidence and guidance. The peer reviewers listed here have reviewed the content at least once during the history of the topic.
Disclosures
Peer reviewer affiliations and disclosures pertain to the time of the review.
参考文献
关键文献
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: esophageal and esophagogastric junction cancers [internet publication].全文
Obermannová R, Alsina M, Cervantes A, et al; ESMO Guidelines Committee. Oesophageal cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022 Oct;33(10):992-1004.全文 摘要
参考文献
A full list of sources referenced in this topic is available to users with access to all of BMJ Best Practice.
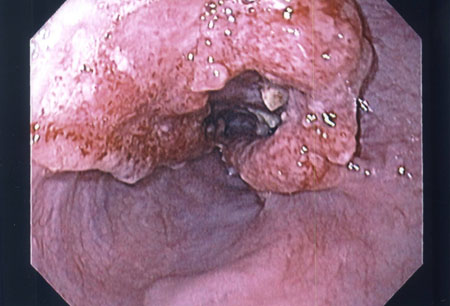
鉴别诊断
- Estenose benigna
- Acalasia
- esôfago de Barrett
更多 鉴别诊断指南
- NCCN clinical practice guidelines in oncology: esophageal and esophagogastric junction cancers
- Oesophageal cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up
更多 指南患者教育信息
Abandono do hábito de fumar
Câncer esofágico
更多 患者教育信息登录或订阅即可浏览 BMJ Best Practice 临床实践完整内容
内容使用需遵循免责声明