Summary
Definition
History and exam
Key diagnostic factors
- presencia de factores de riesgo
- receptor de trasplante de células hematopoyéticas (TCH) alogénico
- llagas dolorosas en la boca de inicio reciente
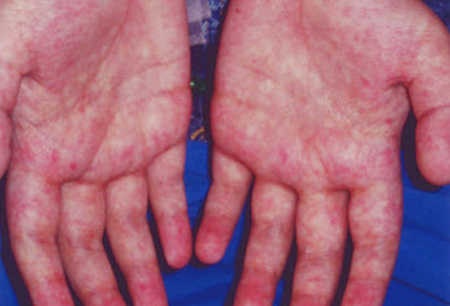
- lesiones cutáneas hiperpigmentadas
- erupción maculopapular difusa
- signos y síntomas genitales
- náuseas, vómitos, dolor abdominal, diarrea profusa y anorexia
Other diagnostic factors
- rigidez o tirantez de las articulaciones
- del día +21 al día +25 después del TCH
- sequedad, aspereza y dolor en los ojos
- ictericia
- hepatomegalia
- esclerodermia
Risk factors
- Incompatibilidad de HLA y donante no emparentado
- EICH aguda previa
- receptor o donante en grupo etario avanzado
- donante mujer con receptor hombre
- donante femenina que ha dado a luz
- tipo y etapa de la enfermedad maligna subyacente
- régimen de radiación de acondicionamiento de alta intensidad
- trasplante de células madre de sangre periférica
- infusión de linfocitos de donante (DLI)
- ausencia de profilaxis o profilaxis insuficiente para la EICH
- raza blanca/negra
- citomegalovirus (CMV) seropositivo
- esplenectomía
- baja puntuación de estado funcional
- estado socioeconómico bajo
Diagnostic tests
1st tests to order
- hemograma completo (HC)
- electrolitos séricos
- pruebas de función hepática
- análisis de orina
- urocultivo
- hemocultivo
- coprocultivo
- estudios de reacción en cadena de la polimerasa (PCR) viral
Tests to consider
- tomografía computarizada (TC) de abdomen
- ultrasonido Doppler del hígado
- biopsia de tejido (piel, tracto gastrointestinal [GI], hígado o pulmón)
- pruebas funcionales respiratorias
- exploración de tórax por TC de alta resolución
- lavado broncoalveolar (LBA) y cultivo
- ecocardiograma
- deglución de bario o endoscopia gastrointestinal superior
- Exploración por tomografía de emisión de positrones con 18F-fluorodesoxiglucosa (FDG-TEP)
Emerging tests
- Biomarcadores séricos
Treatment algorithm
receptor de trasplante de células hematopoyéticas (TCH)
aguda: grado I
aguda: grados II-IV
crónica
Contributors
Authors
Sung Won Choi, MD, MS

Professor
Department of Pediatrics
Division of Pediatric Hematology Oncology/Blood and Marrow Transplantation
University of Michigan
Ann Arbor
MI
Disclosures
SWC is an author of a number of references cited in this topic.
Lyndsey Runaas, MD

Assistant Professor, Hematology and Oncology
Division of Hematology/Oncology
Medical College of Wisconsin
Milwaukee
WI
Disclosures
LR declares that she has no competing interests.
Acknowledgements
Dr Sung Choi and Dr Lyndsey Runaas would like to gratefully acknowledge Dr Pavan Reddy, a previous contributor to this topic.
Disclosures
PR is an author of a number of references cited in this topic.
Peer reviewers
Corey Cutler, MD, MPH, FRCPC
Associate Professor of Medicine
Harvard Medical School
Dana-Farber Cancer Institute
Boston
MA
Disclosures
CC declares that he has no competing interests.
Waseem Qasim, BMedSci (Hons), MBBS, MRCP (UK), MRCPCH, PhD
Senior Lecturer
Institute of Child Health
Consultant in Paediatric Immunology & Bone Marrow Transplantation
Great Ormond Street Hospital
London
UK
Disclosures
WQ declares that he has no competing interests.
Peer reviewer acknowledgements
BMJ Best Practice topics are updated on a rolling basis in line with developments in evidence and guidance. The peer reviewers listed here have reviewed the content at least once during the history of the topic.
Disclosures
Peer reviewer affiliations and disclosures pertain to the time of the review.
References
Key articles
Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015 Mar;21(3):389-401.e1.Full text Abstract
Penack O, Marchetti M, Aljurf M, et al. Prophylaxis and management of graft-versus-host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2024 Feb;11(2):e147-59. Abstract
Dignan FL, Amrolia P, Clark A, et al. Diagnosis and management of chronic graft-versus-host disease. Br J Haematol. 2012 Jul;158(1):46-61.Full text Abstract
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: hematopoietic cell transplantation [internet publication].Full text
Dignan FL, Clark A, Amrolia P, et al. Diagnosis and management of acute graft-versus-host disease. Br J Haematol. 2012 Jul;158(1):30-45.Full text Abstract
Carpenter PA, Kitko CL, Elad S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: V. The 2014 Ancillary Therapy and Supportive Care Working Group report. Biol Blood Marrow Transplant. 2015 Jul;21(7):1167-87.Full text Abstract
Reference articles
A full list of sources referenced in this topic is available to users with access to all of BMJ Best Practice.
Differentials
- Erupción por fármacos
- Erupción cutánea inducida por radiación o quimioterapia
- Gastroenteritis bacteriana
More DifferentialsGuías de práctica clínica
- Prophylaxis and management of graft-versus-host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations
- NCCN clinical practice guidelines in oncology: hematopoietic cell transplantation
More Guías de práctica clínicaCalculadoras
La puntuación de riesgo de EICH aguda refinada de Minnesota
Más CalculadorasInicie sesión o suscríbase para acceder a todo el BMJ Best Practice
El uso de este contenido está sujeto a nuestra cláusula de exención de responsabilidad
