Resumen
Definición
Anamnesis y examen
Principales factores de diagnóstico
- palidez
- equimosis o petequias
Otros factores de diagnóstico
- fatiga
- mareos
- palpitaciones
- disnea
- fiebre e infecciones
- linfadenopatía
- hepatoesplenomegalia
- sangrado mucoso
- masa testicular
- masa cutánea (p. ej., sarcoma mieloide)
- infiltración cutánea
- agrandamiento gingival
- dolor óseo
- síntomas gastrointestinales (por ejemplo, dolor abdominal severo)
- síntomas neurológicos (por ejemplo, dolor de cabeza, confusión)
Factores de riesgo
- mayores de 65 años
- tratamiento previo con quimioterapia
- trastornos hematológicos previos
- Trastornos genéticos hereditarios
- anomalías cromosómicas constitucionales
- exposición a la radiación ionizante
- exposición al benceno
- exposiciones ambientales
- sexo masculino
Pruebas diagnósticas
Primeras pruebas diagnósticas para solicitar
- hemograma completo (HC) con diferencial
- frotis de sangre periférica
- perfil de coagulación
- electrolitos séricos
- ácido úrico sérico
- lactato deshidrogenasa (LDH) sérica
- función renal
- pruebas de función hepática
- evaluación de la médula ósea
- pruebas genéticas
Pruebas diagnósticas que deben considerarse
- pruebas genéticas para el síndrome de predisposición hereditaria a neoplasias malignas hematológicas
- tipificación del antígeno leucocitario humano (HLA)
- Estudios por imágenes del SNC y punción lumbar
- exploración TEP-FDG/TC
- radiografía de tórax
- ecocardiograma
- ventriculografía con radionúclidos
- tipificación del antígeno leucocitario humano (HLA)
Algoritmo de tratamiento
LMA recién diagnosticada: apta para quimioterapia intensiva
LMA recién diagnosticada: no apta para quimioterapia intensiva
leucemia promielocítica aguda (LPMA) de reciente diagnóstico y sin alto riesgo
leucemia promielocítica aguda (LPMA) de alto riesgo recién diagnosticada
remisión completa: LMA
remisión completa: leucemia promielocítica aguda de alto riesgo (LPMA)
LMA en recidiva o refractaria
leucemia promielocítica aguda (LPMA) en recidiva o refractaria
Colaboradores
Autores
Vijaya Raj Bhatt, MBBS, MS
Associate Professor
Section Leader, Malignant Hematology
University of Nebraska Medical Center Division of Hematology-Oncology
Nebraska
NE
Divulgaciones
VRB has participated in a Safety Monitoring Committee for Protagonist Therapeutics. He serves as an Associate Editor for the Elsevier Journal, Current Problems in Cancer. He has received consulting fees from Imugene, Sanofi, and Taiho; research funding (institutional) from Abbvie, Pfizer, Incyte, Jazz, NMDP, MEI Pharma, Sanofi, and Actinium Pharmaceuticals; and drug support (institutional) from Chimerix for a trial.
Prajwal Dhakal, MBBS
Clinical Assistant Professor of Internal Medicine-Hematology, Oncology, and Blood and Marrow Transplantation
University of Iowa
Iowa City
IA
Divulgaciones
PD declares that he has no competing interests.
Agradecimientos
Dr Vijaya Raj Bhatt and Dr Prajwal Dhakal would like to gratefully acknowledge Dr Kavita Raj and Dr Priyanka Mehta, previous contributors to this topic.
Divulgaciones
KR declares that she has no competing interests. PM is an author of a reference cited in this topic.
Revisores por pares
Naveen Premnath, MD
Assistant Professor of Medicine
Division of Hematology, Oncology, and Transplantation
University of Minnesota
Minnesota
MN
Divulgaciones
NP declares that he has no competing interests.
Rebecca Connor, MD
Chief Fellow
Section of Hematology and Oncology
Department of Internal Medicine
Wake Forest University Baptist Medical Center
Winston-Salem
NC
Divulgaciones
RC declares that she has no competing interests.
Roger M. Lyons, MD, FACP
Clinical Professor of Medicine
University of Texas Health Science Center
San Antonio
Cancer Care Network of South Texas
San Antonio
TX
Divulgaciones
RML declares that he has no competing interests.
Shankaranarayana Paneesha, MD, MRCP, FRCPath
Consultant Haematologist
Department of Haematology and Stem Cell Transplantation
Heartlands Hospital
Birmingham
UK
Divulgaciones
SP declares that he has no competing interests.
David Marks, MD, MRCP, MRCPath
Professor of Haematology & Stem Cell Transplantation
Department of Molecular and Cellular Medicine
University of Bristol
UK
Divulgaciones
DM declares that he has no competing interests.
Agradecimiento de los revisores por pares
Los temas de BMJ Best Practice se actualizan de forma continua de acuerdo con los desarrollos en la evidencia y en las guías. Los revisores por pares listados aquí han revisado el contenido al menos una vez durante la historia del tema.
Divulgaciones
Las afiliaciones y divulgaciones de los revisores por pares se refieren al momento de la revisión.
Referencias
Artículos principales
Döhner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022 Sep 22;140(12):1345-77.Texto completo Resumen
National Cancer Comprehensive Network. NCCN guidelines in oncology: acute myeloid leukemia [internet publication].Texto completo
Heuser M, Ofran Y, Boissel N, et al. Acute myeloid leukaemia in adult patients: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020 Jun;31(6):697-712.Texto completo Resumen
Sekeres MA, Guyatt G, Abel G, et al. American Society of Hematology 2020 guidelines for treating newly diagnosed acute myeloid leukemia in older adults. Blood Adv. 2020 Aug 11;4(15):3528-49.Texto completo Resumen
Sanz MA, Fenaux P, Tallman MS, et al. Management of acute promyelocytic leukemia: updated recommendations from an expert panel of the European LeukemiaNet. Blood. 2019 Apr 11;133(15):1630-43.Texto completo Resumen
Artículos de referencia
Una lista completa de las fuentes a las que se hace referencia en este tema está disponible para los usuarios con acceso a todo BMJ Best Practice.
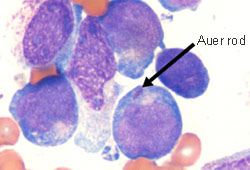
Diferenciales
- Leucemia linfoblástica aguda (LLA)
- Leucemia bifenotípica
- Neoplasias mieloides/linfoides con eosinofilia y genes de fusión de tirosina cinasa (MLNE, por sus siglas en inglés)
Más DiferencialesGuías de práctica clínica
- Suspected cancer: recognition and referral
- NCCN practice guidelines in oncology: hematopoietic cell transplantation
Más Guías de práctica clínicaInicie sesión o suscríbase para acceder a todo el BMJ Best Practice
El uso de este contenido está sujeto a nuestra cláusula de exención de responsabilidad

