Summary
Definition
History and exam
Key diagnostic factors
- presencia de factores de riesgo
Other diagnostic factors
- síntomas constitucionales
- ictericia
- ascitis
- signos de encefalopatía hepática
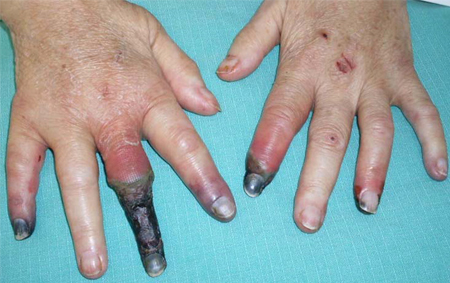
- manifestaciones extrahepáticas
Risk factors
- prácticas médicas inseguras
- consumo de drogas ilícitas por vía intravenosa o intranasal
- transfusión de sangre o trasplante de órganos
- consumo excesivo de alcohol
- polimorfismo del gen de interleucina (IL)-28B
- VIH
- encarcelamiento/institucionalización
- diálisis
- profesión relacionada con la salud
- tatuajes/perforaciones corporales
- acupuntura
- parejas sexuales múltiples
- madre infectada (para el feto)
- sexo masculino
- deficiencia de vitamina D
Diagnostic tests
1st tests to order
- Inmunoanálisis de enzimas de anticuerpos del virus de la hepatitis C (VHC)
- reacción en cadena de la polimerasa (PCR) para el ARN del virus de la hepatitis C (VHC)
- aminotransferasas séricas
Tests to consider
- genotipado viral
- ensayo del antígeno central del virus de la hepatitis C (VHC)
- pruebas no invasivas de fibrosis hepática o elasticidad
- índice de relación aspartato aminotransferasa/plaquetas (APRI)
- biopsia hepática
- pruebas para detectar una coinfección
Treatment algorithm
infección reciente
infección crónica apta para un tratamiento simplificado: cualquier genotipo
infección crónica no apta para tratamiento simplificado y sin tratamiento: genotipo 1a
infección crónica no apta para tratamiento simplificado y sin tratamiento: genotipo 1b
infección crónica no apta para tratamiento simplificado y sin tratamiento: genotipo 2
infección crónica no apta para tratamiento simplificado y sin tratamiento: genotipo 3
infección crónica no apta para tratamiento simplificado y sin tratamiento: genotipo 4
infección crónica no apta para tratamiento simplificado y sin tratamiento: genotipo 5 o 6
infección crónica no apta para el tratamiento simplificado y con experiencia en el tratamiento: todos los genotipos
cirrosis descompensada
Contributors
Authors
Jawad Ahmad, MD, FRCP, FAASLD
Professor of Medicine
Division of Liver Diseases
Mount Sinai Hospital
New York
NY
Disclosures
JA declares that he has no competing interests.
Acknowledgements
Dr Jawad Ahmad would like to gratefully acknowledge Dr Brian L. Pearlman, the previous contributor to this topic.
Disclosures
BLP is on the speakers' bureaus and serves as an advisor to Merck, Gilead, J&J, and AbbVie, and does contracted research with Boehringer Ingelheim, Tibotec/Janssen, Bristol-Myers Squibb, Gilead, and Merck. BLP is also an author of a number of references cited in this topic. BLP wishes to acknowledge Chaithanya Mallikarjun, MD, for her help in writing the original version of the manuscript.
Peer reviewers
AnnMarie Liapakis, MD
Assistant Professor
Digestive Disease and Liver Transplant
Yale University
CT
Disclosures
AL has participated in an advisory board meeting with Gilead and Janssen, and is a primary investigator for Merck’s C-Surfer trial.
Benedict Maliakkal, MRCP
Director of Hepatology and Medical Director of Liver Transplantation
Associate Professor of Medicine
Strong Memorial Hospital
University of Rochester
NY
Disclosures
BM belongs to the paid speaker’s bureau for pharmaceutical companies Gilead (maker of ledipasvir/sofosbuvir), AbbVie (maker of Viekira Pak® - ombitasvir/paritaprevir/ritonavir and dasabuvir), Merck, and Salix.
Sulleman Moreea, FRCS (Glasg), FRCP
Consultant Hepatologist
Bradford Teaching Hospitals Foundation Trust
Bradford
UK
Disclosures
SM has received sponsorship and has sat on advisory boards for BMS, Gilead, AbbVie, and Merck.
Steve Ryder, DM, FRCP
Consultant Hepatologist
Nottingham University Hospitals NHS Trust
UK
Declarações
SR is an advisory board (paid) member for AbbVie, Gilead, Janssen, and MSD.
Créditos aos pareceristas
Os tópicos do BMJ Best Practice são constantemente atualizados, seguindo os desenvolvimentos das evidências e das diretrizes. Os pareceristas aqui listados revisaram o conteúdo pelo menos uma vez durante a história do tópico.
Declarações
As afiliações e declarações dos pareceristas referem--se ao momento da revisão.
Referências
Principais artigos
American Association for the Study of Liver Diseases; Infectious Diseases Society of America. HCV guidance: recommendations for testing, managing, and treating hepatitis C. Dec 2023 [internet publication].Texto completo
Artigos de referência
Uma lista completa das fontes referenciadas neste tópico está disponível para os usuários com acesso total ao BMJ Best Practice.
Diagnósticos diferenciais
- Hepatitis B
- Hepatitis D
- Hepatitis E
Mais Diagnósticos diferenciaisDiretrizes
- Clinical screening and diagnosis for hepatitis C
- Bloodborne viruses in healthcare workers: health clearance and management
Mais DiretrizesFolhetos informativos para os pacientes
Hepatitis C: preguntas para formularle al médico
Hepatitis C: ¿cuáles son las opciones de tratamiento?
Mais Folhetos informativos para os pacientesConectar-se ou assinar para acessar todo o BMJ Best Practice
O uso deste conteúdo está sujeito ao nosso aviso legal