Резюме
Определение
Анамнез и осмотр
Ключевые диагностические факторы
- previous stay in rural west and central Africa (Trypanosoma brucei gambiense)
- previous stay in game parks in east and southern Africa (T b rhodesiense)
- enlarged cervical lymph nodes/Winterbottom sign (T b gambiense)
- chancre (T b rhodesiense)
- disturbances of consciousness and sleep
Другие диагностические факторы
- history of several treatments against malaria with no improvement
- headache
- fever
- fatigue and general malaise
- history of infertility, menstrual disorders, high miscarriage rate (women)
- reduced libido, impotence (men)
- pruritus
- edema
- impaired motor functions
- mental changes
- signs of cardiac failure (T b rhodesiense)
- rash
- hepatosplenomegaly
- sensory disorders
Факторы риска
- exposure to tsetse fly
- living or working in an area with people infected with gambiense trypanosomiasis
- living or working in an area with animals infected with rhodesiense trypanosomiasis
Диагностические исследования
Исследования, которые показаны в первую очередь
- complete blood count
- erythrocyte sedimentation rate
- serum immunoglobulins (Ig)
- rapid diagnostic tests
- card agglutination test for trypanosomiasis (CATT)
- immunofluorescence
- enzyme-linked immunosorbent assay (ELISA)
- chancre aspirate microscopy
- lymph node aspirate microscopy
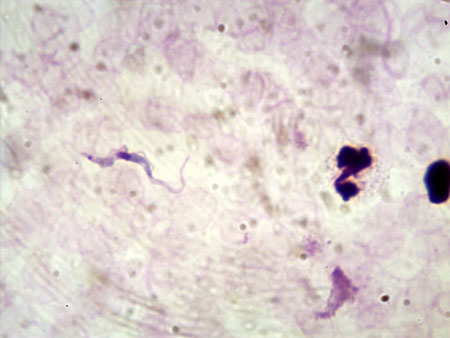
- blood microscopy
- microhematocrit centrifugation technique
- quantitative buffy coat technique
- mini-anion exchange centrifugation technique (mAECT)
Исследования, проведение которых нужно рассмотреть
- electrocardiogram
- cerebrospinal fluid (CSF) white blood cell (WBC) count
- CSF microscopy
- double centrifugation of CSF
- modified single centrifugation of CSF
- CSF protein
Неотложные исследования
- polymerase chain reaction (PCR)
- reverse transcriptase real-time PCR (RT-PCR)
- intrathecal immunoglobulin production
- stage biomarkers
- immune trypanolysis
- T b gambiense inhibition ELISA (g-iELISA)
- MRI brain
Алгоритм лечения
gambiense human African trypanosomiasis (HAT)
rhodesiense human African trypanosomiasis (HAT)
relapse
Составители
Авторы
Veerle Lejon, PhD
Director of Research
Institut de Recherche pour le Développement
Montpellier
France
Раскрытие информации
VL is an author of several references cited in this topic. VL declares that she has no competing interests.
José Ramón Franco, MD, MPH
Medical Officer
Control of Neglected Tropical Diseases
Human African Trypanosomiasis Control Program
World Health Organization
Geneva
Switzerland
Раскрытие информации
JRF is an author of several references cited in this topic. JRF declares that he has no competing interests.
Pere P. Simarro, MD, PhD
Former head of WHO HAT control and surveillance programme
WHO temporary advisor
World Health Organization
Geneva
Switzerland
Раскрытие информации
PPS is an author of several references cited in this topic.
Рецензенты
Sanjeev Krishna, MA (Cantab), BMChB (Oxon), DPhil, FRCP, ScD (Cantab), FMedSci
Professor of Molecular Parasitology and Medicine
Centre for Infection
Division of Cellular and Molecular Medicine
St. George's
University of London
London
UK
Declarações
SK is a consultant for the Foundation for Innovative Diagnostics, a non-profit organization developing diagnostics for neglected diseases such as HAT. SK is an author of a reference cited in this topic.
Mike Barrett, BSc, PhD
Professor
Division of Infection and Immunity
Institute of Biomedical and Life Sciences
The Glasgow Biomedical Research Centre
University of Glasgow
Glasgow
UK
Declarações
MB declares that he has no competing interests.
Créditos aos pareceristas
Os tópicos do BMJ Best Practice são constantemente atualizados, seguindo os desenvolvimentos das evidências e das diretrizes. Os pareceristas aqui listados revisaram o conteúdo pelo menos uma vez durante a história do tópico.
Declarações
As afiliações e declarações dos pareceristas referem--se ao momento da revisão.
Referências
Principais artigos
World Health Organization. Guidelines for the treatment of human African trypanosomiasis. Jun 2024 [internet publication].Texto completo
Centers for Disease Control and Prevention. Clinical care of human African trypanosomiasis. Apr 2024 [internet publication].Texto completo
Artigos de referência
Uma lista completa das fontes referenciadas neste tópico está disponível para os usuários com acesso total ao BMJ Best Practice.
Diagnósticos diferenciais
- Malaria
- Typhoid fever
- Relapsing fever
Mais Diagnósticos diferenciaisDiretrizes
- Guidelines for the treatment of human African trypanosomiasis
- Clinical care of human African trypanosomiasis
Mais DiretrizesConectar-se ou assinar para acessar todo o BMJ Best Practice
O uso deste conteúdo está sujeito ao nosso aviso legal