Summary
Definition
History and exam
Key diagnostic factors
- hematuria (visible or nonvisible)
Other diagnostic factors
- urinary frequency
- dysuria
Risk factors
- tobacco exposure
- exposure to chemical carcinogens
- age >65 years
- pelvic radiation
- cyclophosphamide or ifosfamide use
- Schistosoma infection
- male sex
- chronic bladder inflammation
- genetic predisposition
- diabetes mellitus
Diagnostic tests
1st tests to order
- urinalysis
Tests to consider
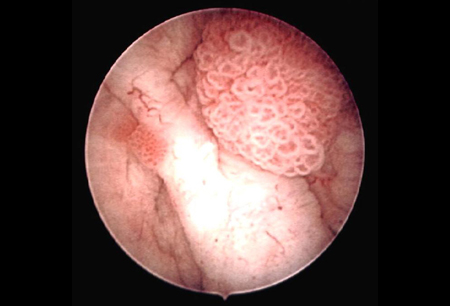
- cystoscopy
- urine cytology
- CT urogram
- MR urogram
- renal and bladder ultrasound
- surgery
- CBC
- chemistry profile (including alkaline phosphatase)
- chest x-ray
- CT abdomen and pelvis
- MRI abdomen and pelvis
- fluorodeoxyglucose (FDG)-PET/CT
- bone scan
- urine biomarkers
- genetic testing
Treatment algorithm
nonmuscle-invasive tumors
locally invasive tumors
metastatic disease
Contributors
Authors
Alberto Martini, MD
Assistant Professor, Director of Research
Department of Urology
University of Cincinnati College of Medicine
Cincinnati
OH
Disclosures
AM declares that he has no competing interests.
Acknowledgements
Dr Alberto Martini would like to gratefully acknowledge Dr Joshua J. Meeks, Dr David VanderWeele, Dr Sarah E. Fenton, Dr Donald Lamm and Dr Mary Heeley, previous contributors to this topic.
Disclosures
JJM is a consultant for Merck, AstraZeneca, Incyte, Janssen, BMS, UroGen, Prokarium, Imvax, Pfizer, and Seagen/Astellas. He has received research funding from the VHA, NIH, and DoD; compensation for talks/educational courses from the AUA, OncLive, Olympus, and UroToday; and clinical trial support from SWOG, Genentech, Merck, AstraZeneca, and Incyte. JJM holds patents on T1 and TCGA classifiers; these are not currently available for use in clinical practice. JJM is an author of a reference cited in this topic. DVW declares that he has received payments from Clovis Oncology, Exelixis, Janssen, and Bayer for advisory boards; research payments from AstraZeneca for clinical trials; payments from Astellas and Myovant for lectures; and payments from Exelixis and AstraZeneca for travel and dinner costs. SEF declares that she has no competing interests. DL is an author of a number of references cited in this topic. MH declares that she has no competing interests.
Peer reviewers
Junaid Masood, MBBS, FRCS (Eng), MSc (Urol), FRCS (Urol)
Consultant Urological Surgeon
Homerton University Hospital NHS Foundation Trust
London
UK
Disclosures
JM declares that he has no competing interests.
Hugh Mostafid, MD
Consultant Urologist
North Hampshire Hospital
Basingstoke
UK
Disclosures
HM has received honoraria from GE Healthcare and Kyowa Kirin UK.
Thomas Guzzo, MD
Clinical Instructor of Urology
The James Buchanan Brady Urologic Institute
The Johns Hopkins Medical Institutions
Baltimore
MD
Disclosures
TG declares that he has no competing interests.
Amir Kaisary, MD, MA, ChM, FRCS
Consultant Urological Surgeon
Honorary Senior Lecturer
Department of Urology
The Royal Free & University College Medical School
London
UK
Disclosures
AK declares that he has no competing interests.
Peer reviewer acknowledgements
BMJ Best Practice topics are updated on a rolling basis in line with developments in evidence and guidance. The peer reviewers listed here have reviewed the content at least once during the history of the topic.
Disclosures
Peer reviewer affiliations and disclosures pertain to the time of the review.
References
Key articles
European Association of Urology. Non-muscle-invasive bladder cancer (TaT1 and CIS). 2025 [internet publication].Full text
European Association of Urology. Muscle-invasive and metastatic bladder cancer. 2025 [internet publication].Full text
American Urological Association. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO joint guideline. 2024 [internet publication].Full text
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: bladder cancer [internet publication].Full text
American Urological Association. Treatment of non-metastatic muscle-invasive bladder cancer: AUA/ASCO/ASTRO/SUO guideline. Apr 2024 [internet publication].Full text
Reference articles
A full list of sources referenced in this topic is available to users with access to all of BMJ Best Practice.
Differentials
- Benign prostatic hyperplasia (BPH)
- Hemorrhagic cystitis
- Acute prostatitis
More DifferentialsGuidelines
- NCCN clinical practice guidelines in oncology: bladder cancer
- Canadian Urological Association guideline: muscle-invasive bladder cancer
More GuidelinesPatient information
Bladder cancer
More Patient informationLog in or subscribe to access all of BMJ Best Practice
Use of this content is subject to our disclaimer