小结
定义
病史和体格检查
关键诊断因素
- periorbital/retro-ocular pain
- loss of visual acuity with scotoma
- color desaturation/loss of color vision
- relative afferent papillary defect (RAPD)
其他诊断因素
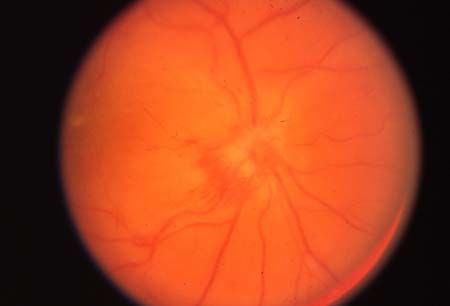
- optic disk swelling
- neurologic abnormalities of multiple sclerosis
- Uhthoff phenomenon
- phosphenes
- Pulfrich phenomenon
- perivenous sheathing
危险因素
- age 30 to 50 years
- female sex
- white ethnicity
- HLA-DRB1*1501 genotype
- risk factors for multiple sclerosis (MS; Epstein-Barr virus [EBV] infection, vitamin D deficiency, and smoking)
- childhood in higher latitudes
- presence of autoimmune disease
- exposure to infectious diseases, such as Lyme disease and syphilis
诊断性检查
首要检查
- MRI - brain
- CBC
- ESR
- C-reactive protein
- venereal disease research laboratory (VDRL)
- aquaporin-4 (AQP4) antibody, myelin oligodendrocyte glycoprotein (MOG) antibody, and collapsin response mediator protein 5 (CRMP5) antibody
- uric acid
- serum ACE
- ANA
- B12 and folate
需考虑的检查
- Lyme titer
- cerebrospinal fluid analysis
治疗流程
acute episode
coexisting inflammatory diseases (e.g., NMOSD, MOGAD, SLE or sarcoidosis)
撰稿人
作者
Cris S. Constantinescu, MD, PhD
Chair of Neurology Emeritus
Academic Unit of Mental Health and Clinical Neuroscience
University of Nottingham
Nottingham
UK
Attending Neurologist
Professor of Neurology
Cooper Neuroscience Institute
Camden
New Jersey
利益声明
CSC has received research support or consultancy fees from Biogen, GW, Merck, Morphosys, Novartis, Roche, Sanofi-Pasteur, MSD, and Teva. CSC is an author of a number of references cited in this topic.
Thomas M. Bosley, MD

Knights Templar Eye Foundation Professor of Ophthalmology
Wilmer Eye Institute
Johns Hopkins University
Baltimore
MD
利益声明
TMB declares that he has no competing interests.
同行评议者
Gus Gazzard, MA, MBBChir, MD, FRCOphth
Consultant Ophthalmic Surgeon
King's College Hospital
Honorary Research Fellow
Institute of Ophthalmology
London
UK
利益声明
GG declares that he has no competing interests.
John Selhorst, MD
Professor
Department of Neurology and Psychiatry
Saint Louis University
St. Louis
MO
利益声明
JS declares that he has no competing interests.
Efstratios Mendrinos, MD
Department of Ophthalmology
Geneva University Hospitals
Geneva
Switzerland
利益声明
EM declares that he has no competing interests.
Jonathan Smith, MD
Specialist Registrar in Ophthalmology
Royal Victoria Infirmary
Newcastle
UK
利益声明
JS declares that he has no competing interests.
Peter Sguigna, MD
Assistant Professor
Department of Neurology
The University of Texas
Southwestern Medical Center
Dallas
TX
利益声明
PS has received grant support from National Multiple Sclerosis Society, International Progressive Multiple Sclerosis Alliance, National Institute of Health (NIH), Center for Translational Medicine, Physician Scientist Training Program, and Department of Defense (DOD). He has received research support from Genentech, Clene Nanomedicine, and Patient Centered Outcomes Research Institute. He has received consulting fees from Medical Logix LLC, Genentech, EMD Serono, Horizon Therapeutics, and Bristol Myers Squibb. He is the inventor on a patent for OCT technology that is held by UT Southwestern Medical Center.
Peer reviewer acknowledgements
BMJ Best Practice topics are updated on a rolling basis in line with developments in evidence and guidance. The peer reviewers listed here have reviewed the content at least once during the history of the topic.
Disclosures
Peer reviewer affiliations and disclosures pertain to the time of the review.
参考文献
关键文献
Petzold A, Fraser CL, Abegg M, et al. Diagnosis and classification of optic neuritis. Lancet Neurol. 2022 Dec;21(12):1120-34. 摘要
American Academy of Ophthalmology. Optic neuritis - 2012. Jul 2012 [internet publication].全文
Beck RW, Cleary PA, Anderson MM Jr, et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. The Optic Neuritis Study Group. N Engl J Med. 1992 Feb 27;326(9):581-8. 摘要
Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018 Feb;17(2):162-73.全文 摘要
Sellner J, Boggild M, Clanet M, et al. EFNS guidelines on diagnosis and management of neuromyelitis optica. Eur J Neurol. 2010 Aug;17(8):1019-32.全文 摘要
Gal RL, Vedula SS, Beck R. Corticosteroids for treating optic neuritis. Cochrane Database Syst Rev. 2015 Aug 14;(8):CD001430.全文 摘要
参考文献
A full list of sources referenced in this topic is available to users with access to all of BMJ Best Practice.
鉴别诊断
- Lyme disease
- Syphilis
- HIV infection
更多 鉴别诊断指南
- Optic neuritis
- Guidelines on diagnosis and management of neuromyelitis optica
更多 指南患者教育信息
Lyme disease
Multiple sclerosis
更多 患者教育信息登录或订阅即可浏览 BMJ Best Practice 临床实践完整内容
内容使用需遵循免责声明