Resumo
Definição
História e exame físico
Principais fatores diagnósticos
- polyuria
- polydipsia
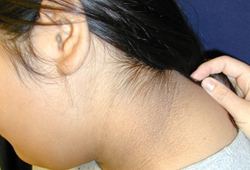
- acanthosis nigricans
- nocturia
Outros fatores diagnósticos
- hypertension
- yeast infections
- skin infections
- urinary tract infections
- fatigue
- blurred vision
- weight loss
Fatores de risco
- obesity
- genetic predisposition/family history
- African-American, Hispanic, American-Indian, and Asian or Pacific Islander
- puberty
- female sex
- diabetic in-utero environment
- small for gestational age
- rapid growth in infancy
- bottle feeding
- high protein intake in infancy
- polycystic ovaries
- intramyocellular lipid content
- fat deposition in the liver
- learning disability
Investigações diagnósticas
Primeiras investigações a serem solicitadas
- urine dipstick
- random plasma glucose
- fasting plasma glucose
- HbA1c
- autoantibodies to insulin, islet antigen (IA-2), glutamic acid decarboxylase (GAD), and zinc transporter 8 (ZnT8)
Investigações a serem consideradas
- 2-hour plasma glucose
- random C-peptide
Algoritmo de tratamento
ketoacidosis or hyperglycemic hyperosmolar state (HHS)
HbA1c <8.5%: no acidosis or ketosis
HbA1c ≥8.5%: no acidosis with or without ketosis
Colaboradores
Autores
Jennifer Miller, MD

Professor
Department of Pediatrics
University of Florida
Gainesville
FL
Declarações
JM declares that she has received research funding from Soleno Therapeutics, Harmony Biosciences, and Rhythm Pharmaceuticals. JM is an author of a reference cited in this topic.
Chelsea Zimmerman, MD
Clinical Physician
Pediatric Endocrinology
Endocrinology and Metabolism of East Alabama
Opelika
AL
Declarações
CZ declares that she has no competing interests.
Agradecimentos
Dr Jennifer Miller and Dr Chelsea Zimmerman would like to gratefully acknowledge Dr Arlan Rosenbloom and Dr Paul Hiers, previous contributors to this topic.
Declarações
AR is an author of a number of references cited in this topic. Unfortunately, we have since been made aware that AR is deceased. PH declares that he has no competing interests.
Revisores
Philip Zeitler, MD, PhD
Professor of Pediatrics and Clinical Science
University of Colorado School of Medicine
Aurora
CO
Declarações
PZ declares that he has no competing interests.
Julian P. Hamilton-Shield, MB, ChB, MD(Bristol), MRCP, FRCPCH, FRCPCH
Professor
Diabetes and Metabolic Endocrinology
School of Clinical Sciences
University of Bristol
UK
Declarações
In the past, JPHS received speaking honoraria from Sanofi-Aventis, Roche, Abbott, Novo Nordisk, and Nutricia. None of these talks were directly pertinent to the treatment of type 2 diabetes.
Kristen Nadeau, MD
Professor of Pediatric Endocrinology
University of Colorado School of Medicine
Aurora
CO
Disclosures
KN declares that she has no competing interests.
Dennis Styne, MD
Professor of Pediatrics
Rumsey Chair of Pediatric Endocrinology
University of California
Sacramento
CA
Disclosures
DS declares that he has no competing interests.
Peer reviewer acknowledgements
BMJ Best Practice topics are updated on a rolling basis in line with developments in evidence and guidance. The peer reviewers listed here have reviewed the content at least once during the history of the topic.
Disclosures
Peer reviewer affiliations and disclosures pertain to the time of the review.
References
Key articles
American Diabetes Association. Standards of care in diabetes - 2025. Diabetes care. 2025 Jan;48(suppl 1):S1-352.Full text
Shah AS, Barrientos-Pérez M, Chang N, et al. ISPAD clinical practice consensus guidelines 2024: type 2 diabetes in children and adolescents. Horm Res Paediatr. 2024;97(6):555-83.Full text Abstract
Glaser N, Fritsch M, Priyambada L, et al. ISPAD clinical practice consensus guidelines 2022: diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatr Diabetes. 2022 Nov;23(7):835-56. Abstract
Reference articles
A full list of sources referenced in this topic is available to users with access to all of BMJ Best Practice.
Differentials
- Impaired glucose tolerance
- Type 1 diabetes mellitus (T1DM)
- Monogenic diabetes: maturity-onset diabetes of the young
More DifferentialsGuidelines
- Standards of care in diabetes - 2025
- Type 2 diabetes in children and adolescents
More GuidelinesPatient information
Diabetes type 2: should I take insulin?
Diabetes type 2: what are the treatment options?
More Patient informationLog in or subscribe to access all of BMJ Best Practice
Use of this content is subject to our disclaimer