Resumo
Definição
História e exame físico
Principais fatores diagnósticos
- skin lesions
Outros fatores diagnósticos
- family history
- joint swelling or pain
Fatores de risco
- genetic
- infection
- local trauma
- medications
- stress
- smoking
- ethnicity
- alcohol
- greater body mass index (BMI)
Investigações diagnósticas
Primeiras investigações a serem solicitadas
- clinical diagnosis
Investigações a serem consideradas
- skin biopsy
Algoritmo de tratamento
plaque
guttate
pustular
Colaboradores
Autores
Andrea Chiricozzi, MD
Assistant Professor
University of Pisa
Consultant Dermatologist
University Hospital of Pisa
Pisa
Italy
Declarações
AC has served as an advisory board member and consultant and has received fees and speaker's honoraria or has participated in clinical trials for AbbVie, Almirall, Biogen, Fresenius Kabi, Leo Pharma, Lilly, Janssen, Novartis, Sanofi Genzyme, and UCB Pharma; AC is also an author of a reference cited in this topic.
Agradecimentos
Dr Andrea Chiricozzi would like to gratefully acknowledge Dr April W. Armstrong and Dr Tsu-Yi Chuang, previous contributors to this topic.
Declarações
TC declares that he has no competing interests. AWA is an investigator and consultant for Abbott, Amgen, and Janssen; AWA is also an author of references cited in this topic.
Revisores
David Burden, MD, FRCP
Western Infirmary, Glasgow
Consultant Dermatologist Dermatology
Glasgow
UK
Declarações
DB has been reimbursed as a consultant, researcher, and lecturer for Abbott, Leo, Pfizer, Merck, Janssen-Cilag, and Novartis.
Paradi Mirmirani, MD
Physician
Department of Dermatology
Kaiser Permanente Vallejo Medical Center
Vallejo
CA
Declarações
PM declares that she has no competing interests.
Créditos aos pareceristas
Os tópicos do BMJ Best Practice são constantemente atualizados, seguindo os desenvolvimentos das evidências e das diretrizes. Os pareceristas aqui listados revisaram o conteúdo pelo menos uma vez durante a história do tópico.
Declarações
As afiliações e declarações dos pareceristas referem--se ao momento da revisão.
Referências
Principais artigos
Smith CH, Yiu ZZN, Bale T, et al. British Association of Dermatologists guidelines for biologic therapy for psoriasis 2020: a rapid update. Br J Dermatol. 2020 Oct;183(4):628-37.Texto completo Resumo
Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology – National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019 Sep;81(3):775-804.Texto completo Resumo
Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology - National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020 Jun;82(6):1445-86.Texto completo Resumo
National Institute for Health and Care Excellence. Psoriasis: assessment and management. September 2017 [internet publication].Texto completo Resumo
Artigos de referência
Uma lista completa das fontes referenciadas neste tópico está disponível para os usuários com acesso total ao BMJ Best Practice.
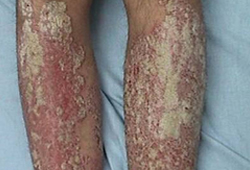
Diagnósticos diferenciais
- Eczema
- Pityriasis rosea
- Seborrheic dermatitis
Mais Diagnósticos diferenciaisDiretrizes
- Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures
- EuroGuiDerm guideline on the systemic treatment of psoriasis vulgaris – part 2: specific clinical and comorbid situations
Mais DiretrizesFolhetos informativos para os pacientes
Psoriasis: what is it?
Psoriasis: what are the treatment options?
Mais Folhetos informativos para os pacientesCalculadoras
PASI Psoriasis Area and Severity Index
Mais CalculadorasConectar-se ou assinar para acessar todo o BMJ Best Practice
O uso deste conteúdo está sujeito ao nosso aviso legal