Summary
Definition
History and exam
Key diagnostic factors
- diarrhea
- weight loss
- arthralgia
- supranuclear ophthalmoplegia
Other diagnostic factors
- abdominal pain
- lymphadenopathy
- fever
- steatorrhea
- anemia
- skin darkening
- confusion, memory impairment, altered level of consciousness, or dementia
- apathy
- anxiety, depression, hypomania, psychosis, change in personality
- myoclonic signs
- seizures
- nystagmus
- brisk reflexes, extensor plantar responses, weakness predominating in arm extensors and leg flexors, hypertonia
- amenorrhea, polydipsia, hyperphagia, decreased libido
- ataxia
- headaches
- oculomasticatory and oculofacioskeletal myorhythmias
- hemiparesis
- cranial nerve involvement
- extrapyramidal movement disorder
- peripheral neuropathies
Risk factors
- age >50 years
- male sex
- genetic factors
Diagnostic tests
1st tests to order
- CBC
- serum albumin
- serum CRP
- serum ESR
- upper GI endoscopy
- Periodic acid-Schiff (PAS) staining of duodenal biopsies
- PCR
- Tropheryma whipplei-specific immunohistochemistry
Emerging tests
- electron microscopy
- culture
- serology
Treatment algorithm
patients without CNS involvement
patients with CNS involvement
Contributors
Autores
Thomas Schneider, MD, PhD
Professor
Medical Department I
Charité - University Medicine Berlin
CBF
Berlin
Germany
Declarações
TS is an author of a number of references cited in this topic.
Verena Moos, PhD
Scientist
Medical Department I
Charité - University Medicine Berlin
CBF
Berlin
Germany
Declarações
VM is an author of a number of references cited in this topic.
Revisores
Stephen G. Baum, MD
Professor of Medicine
Department of Microbiology and Immunology
Albert Einstein College of Medicine
Bronx
NY
Declarações
SGB declares that he has no competing interests.
Chris Huston, MD
Assistant Professor of Medicine
Division of Infectious Diseases
University of Vermont
Burlington
VT
Declarações
CH declares that he has no competing interests.
Créditos aos pareceristas
Os tópicos do BMJ Best Practice são constantemente atualizados, seguindo os desenvolvimentos das evidências e das diretrizes. Os pareceristas aqui listados revisaram o conteúdo pelo menos uma vez durante a história do tópico.
Declarações
As afiliações e declarações dos pareceristas referem--se ao momento da revisão.
Referências
Principais artigos
Schneider T, Moos V, Loddenkemper C, et al. Whipple's disease: new aspects of pathogenesis and treatment. Lancet Infect Dis. 2008 Mar;8(3):179-90. Resumo
Fenollar F, Puechal X, Raoult D. Whipple's disease. N Engl J Med. 2007 Jan 4;356(1):55-66.
Dobbins WO. Whipple's disease. Springfield, IL: Thomas; 1987.
Louis ED, Lynch T, Kaufmann P, et al. Diagnostic guidelines in central nervous system Whipple's disease. Ann Neurol. 1996 Oct;40(4):561-8. Resumo
Feurle GE, Junga NS, Marth T. Efficacy of ceftriaxone or meropenem as initial therapies in Whipple's disease. Gastroenterology. 2010 Feb;138(2):478-86; quiz 11-2.Texto completo Resumo
Feurle GE, Moos V, Bläker H, et al. Intravenous ceftriaxone, followed by 12 or three months of oral treatment with trimethoprim-sulfamethoxazole in Whipple's disease. J Infect. 2013 Mar;66(3):263-70. Resumo
Artigos de referência
Uma lista completa das fontes referenciadas neste tópico está disponível para os usuários com acesso total ao BMJ Best Practice.
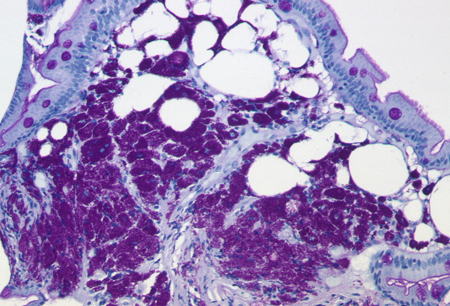
Diagnósticos diferenciais
- Seronegative rheumatoid arthritis
- Sarcoidosis
- Celiac disease
Mais Diagnósticos diferenciaisFolhetos informativos para os pacientes
Diarrhea in adults
Diarrhea in children
Mais Folhetos informativos para os pacientesConectar-se ou assinar para acessar todo o BMJ Best Practice
O uso deste conteúdo está sujeito ao nosso aviso legal