Summary
Definição
História e exame físico
Principais fatores diagnósticos
- jugular venous distention
- lower extremity edema
- history of a chronic inflammatory condition, chronic infection, familial periodic fever syndrome
- history of monoclonal gammopathy of undetermined significance (MGUS)
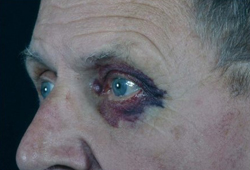
- periorbital purpura
- eyelid petechiae
- macroglossia
Outros fatores diagnósticos
- fatigue
- unexplained weight loss
- dyspnea on exertion
- peripheral neuropathy
- autonomic neuropathy
- claudication
- nausea or vomiting
- abdominal cramps
- alternating bowel habit
- steatorrhea
- lightheaded
- submandibular salivary gland enlargement
- hepatomegaly
- shoulder pad sign
- diffuse muscular weakness
- orthostatic hypotension
- carpal tunnel syndrome
- musculoskeletal disorders
Fatores de risco
- monoclonal gammopathy of undetermined significance (MGUS)
- inflammatory polyarthropathy
- chronic infections
- inflammatory bowel disease
- familial periodic fever syndromes
- Castleman disease
Investigações diagnósticas
Primeiras investigações a serem solicitadas
- serum immunofixation electrophoresis
- urine immunofixation electrophoresis (using 24-hour urine collection)
- serum free light chain assay
- CBC with differential
- peripheral blood smear
- serum quantitative immunoglobulins
- serum protein electrophoresis
- comprehensive metabolic profile
- urine protein electrophoresis (using 24-hour urine collection)
- 24-hour total urine protein
- creatinine clearance
- orthostatic vital sign assessment
- tissue aspiration and biopsy
- fluorescence in situ hybridization (FISH)
Investigações a serem consideradas
- mass spectrometry
- immunohistochemical studies
- immuno-electron microscopy
- genetic testing
- serum troponin T or I
- N-terminal pro-B-type natriuretic peptide (NT‐proBNP)
- lipid panel
- coagulation studies
- ECG
- echocardiogram (with tissue Doppler and global longitudinal strain)
- cardiac MRI (CMR)
- cardiac scintigraphy
- electromyogram/nerve conduction studies
- endocrine tests
- pulmonary function tests
- computed tomography (CT) scan
- fluorodeoxyglucose positron emission tomography (FDG-PET)/CT
- skeletal survey
- abdominal ultrasound
- gastric emptying scan
- upper and lower endoscopy
- 123I-labeled serum amyloid P (SAP) scintigraphy
- 6-minute walk test
Algoritmo de tratamento
immunoglobulin light chain (AL) amyloidosis
AA amyloidosis (nonfamilial)
familial periodic fever syndromes
transthyretin (TTR) amyloidosis
refractory or relapsed AL amyloidosis
Colaboradores
Autores
Morie A. Gertz, MD, MACP

Seidler Jr. Professor of Medicine
Consultant in Hematology
Chair Emeritus of the Department of Medicine
Mayo Distinguished Clinician
Mayo Clinic College of Medicine
Rochester
MN
Declarações
MAG has received personal fees from AbbVie and Arcellx for a Data Safety Monitoring board, personal fees from Ionis/Akcea, Prothena, Sanofi, and Janssen, and fees from Johnson & Johnson. MAG has received honoraria from Alnylam, AstraZeneca, Medscape, Dava Oncology, and Alexion. MAG is an author of several references cited in this topic.
Revisores
Donna Reece, MD
Associate Professor of Medicine
Director
Program for Multiple Myeloma and Related Diseases
Princess Margaret Hospital
Toronto
Ontario
Canada
Declarações
DR has been reimbursed by Millennium Pharmaceuticals, Inc and Johnson & Johnson, the manufacturers of bortezomib, for attending several conferences, for speaking at educational meetings, and for consulting work. She has also been reimbursed by Celgene, the manufacturer of lenalidomide and thalidomide, for attending several symposia and serving as a speaker.
Jeffrey Zonder, MD
Assistant Professor of Medicine and Oncology
Division of Hematology/Oncology
Wayne State University School of Medicine
Barbara Ann Karmanos Cancer Institute
Detroit
MI
利益声明
JZ declares that he has no competing interests.
Peer reviewer acknowledgements
BMJ Best Practice topics are updated on a rolling basis in line with developments in evidence and guidance. The peer reviewers listed here have reviewed the content at least once during the history of the topic.
Disclosures
Peer reviewer affiliations and disclosures pertain to the time of the review.
参考文献
关键文献
Writing Committee, Kittleson MM, Ambardekar AV, et al. Transthyretin cardiac amyloidosis evaluation and management: 2025 ACC concise clinical guidance. J Am Coll Cardiol. 2026 Feb 10;87(5):549-65.全文 摘要
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: systemic light chain amyloidosis [internet publication].全文
Muchtar E, Dispenzieri A, Gertz MA, et al. Treatment of AL amyloidosis: Mayo stratification of myeloma and risk-adapted therapy (mSMART) consensus statement 2020 update. Mayo Clin Proc. 2021 Jun;96(6):1546-77.全文 摘要
Wechalekar AD, Cibeira MT, Gibbs SD, et al. Guidelines for non-transplant chemotherapy for treatment of systemic AL amyloidosis: EHA-ISA working group. Amyloid. 2023 Mar;30(1):3-17.全文 摘要
Sanchorawala V, Boccadoro M, Gertz M, et al. Guidelines for high dose chemotherapy and stem cell transplantation for systemic AL amyloidosis: EHA-ISA working group guidelines. Amyloid. 2022 Mar;29(1):1-7. 摘要
参考文献
A full list of sources referenced in this topic is available to users with access to all of BMJ Best Practice.
鉴别诊断
- Hypertrophic cardiomyopathy (HCM)
- Membranous glomerulopathy
- Monoclonal gammopathy of undetermined significance (MGUS)-associated neuropathy
更多 鉴别诊断指南
- NCCN clinical practice guidelines in oncology: systemic light chain amyloidosis
- NCCN clinical practice guidelines in oncology: hematopoietic cell transplantation (HCT)
更多 指南登录或订阅即可浏览 BMJ Best Practice 临床实践完整内容
内容使用需遵循免责声明