Summary
Definition
History and exam
Key diagnostic factors
- rectal bleeding
- diarrhea
- blood in stool
Other diagnostic factors
- abdominal pain
- arthritis and spondylitis
- malnutrition
- abdominal tenderness
- fever
- weight loss
- constipation
- skin rash
- uveitis and episcleritis
- pallor
Risk factors
- family history of inflammatory bowel disease
- human leukocyte antigen-B27
- infection
- use of nonsteroidal anti-inflammatory drugs (NSAIDs)
- not smoking or former smoker
Diagnostic tests
1st tests to order
- stool studies for infective pathogens
- fecal calprotectin (FC)
- CBC
- comprehensive metabolic panel (including LFTs)
- erythrocyte sedimentation rate (ESR)
- CRP
- plain abdominal radiograph
- flexible sigmoidoscopy
- colonoscopy
- biopsies
Tests to avoid
- serologic antibody testing
Tests to consider
- CT scan
- intestinal ultrasound
Treatment algorithm
acute severe ulcerative colitis (UC)
moderate-to-severe disease
mild disease
disease in remission
Contributors
Authors
Andrew Poullis, BSc, MBBS, MD, FRCP
Consultant Gastroenterologist
Department of Gastroenterology
St George’s Hospital
London
UK
Disclosures
AP is an author of a reference cited in this topic.
Sailish Honap, MBChB (Hons), MRes, MRCP (UK)
Consultant Gastroenterologist
Department of Gastroenterology
St George’s Hospital
London
UK
Disclosures
SH has received honoraria from Pfizer (speaker, consultant, advisory board member, and received travel grants), Janssen (speaker fees, travel grants), AbbVie (speaker fees, consultant, travel grants), Takeda (speaker fees), Ferring (travel grants), Pharmacosmos (travel grants), Galapagos (travel grants).
Kamal Patel,
Disclosures
all disclosures
Aaron Bhakta, BMedSci (Hons), BMBS, MSc, MRCP
Clinical Fellow in Gastroenterology
Department of Gastroenterology
St George’s Hospital
London
UK
Disclosures
AB declares that he has no competing interests.
Acknowledgements
Dr Hesam A. Nooredinvand, and Dr Andrew Poullis would like to gratefully acknowledge Dr Ben Shandro, Dr Anet Soubieres, Dr Rhys Hewett, Dr Willem J.S. de Villiers, and Dr Houssam Mardini, previous contributors to this topic.
Disclosures
BS, AS, RH, WJSD, and HM declare that they have no competing interests.
Peer reviewers
Stephen B Hanauer, MD
Professor of Medicine
Gastroenterology and Hepatology
Northwestern University
Evanston
IL
Disclosures
SW declares that he has no competing interests.
Peer reviewer acknowledgements
BMJ Best Practice topics are updated on a rolling basis in line with developments in evidence and guidance. The peer reviewers listed here have reviewed the content at least once during the history of the topic.
Disclosures
Peer reviewer affiliations and disclosures pertain to the time of the review.
References
Key articles
World Gastroenterology Organisation. Global guidelines: inflammatory bowel disease. Aug 2015 [internet publication].Full text
Rubin DT, Ananthakrishnan AN, Siegel CA, et al. ACG clinical guideline update: ulcerative colitis in adults. Am J Gastroenterol. 2025 Jun 3;120(6):1187-224.Full text Abstract
Moran GW, Gordon M, Sinopolou V, et al. British Society of Gastroenterology guidelines on inflammatory bowel disease in adults: 2025. Gut. 2025 Jun 23;74(Suppl 2):s1-101.Full text Abstract
Singh S, Loftus EV Jr, Limketkai BN, et al. AGA living clinical practice guideline on pharmacological management of moderate-to-severe ulcerative colitis. Gastroenterology. 2024 Dec;167(7):1307-43.Full text Abstract
Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. 2017 Jul 1;11(7):769-84.Full text Abstract
National Institute for Health and Care Excellence (UK). Ulcerative colitis: management. May 2019 [internet publication].Full text
Reference articles
A full list of sources referenced in this topic is available to users with access to all of BMJ Best Practice.
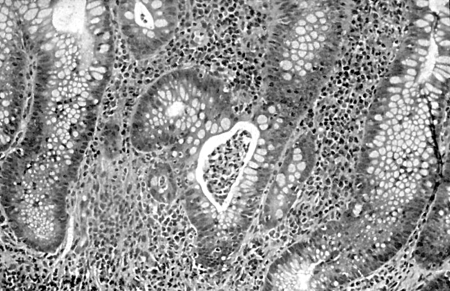
Differentials
- Crohn disease
- Indeterminate colitis
- Radiation colitis
More DifferentialsGuidelines
- Preventive care in inflammatory bowel disease
- Management of inflammatory bowel disease in adults
More GuidelinesPatient information
Ulcerative colitis: what is it?
Ulcerative colitis: what are the treatment options?
More Patient informationVideos
Venepuncture and phlebotomy: animated demonstration
More videosLog in or subscribe to access all of BMJ Best Practice
Use of this content is subject to our disclaimer