Resumo
Definição
História e exame físico
Principais fatores diagnósticos
- number of cigarettes per day
- time to first cigarette (TTFC)
- use of alternative tobacco and nicotine delivery products
- history of substance use disorder (SUD)
- pregnancy or breastfeeding
Outros fatores diagnósticos
- history of depression
- history of schizophrenia
- seizure disorder
- hypertension
- unstable cardiac disease
- ventricular arrhythmia
- asthma
- chronic obstructive pulmonary disease (COPD)
- temporomandibular joint or dental disorder
Fatores de risco
- age <24 years
- low socioeconomic status
- history of mental illness or substance use disorder
- history of HIV/AIDS
- use of alternative tobacco and nicotine delivery products
- genetics
Investigações diagnósticas
Primeiras investigações a serem solicitadas
- self-report of smoking status
- Fagerström Test For Nicotine Dependence (FTND)
- Heaviness of Smoking Index (HSI)
Investigações a serem consideradas
- carbon monoxide (CO) monitoring
- cotinine level
Algoritmo de tratamento
hospitalized active smokers
active smokers ready to quit: adults (not pregnant/breastfeeding)
active smokers ready to quit: pregnant/breastfeeding women or adolescents
active smokers not ready to quit
Colaboradores
Autores
Franck F. Rahaghi, MD, MHS, FCCP
Chairman
Pulmonary Department
Director
Pulmonary Education and Rehabilitation
Cleveland Clinic
Weston
FL
Declarações
FFR is a consultant and speaker for Bayer, United Therapeutics, Janssen-PH, Merck, Boehringer Ingleheim, Takeda, and Talecris. He is involved in industry-sponsored research with Bayer, Janssen-PH, Gossamer Bio, and Bellerophon.
Samuel Gurevich, MD, FCCP
Pulmonary Department
Director
Respiratory Therapy
Cleveland Clinic
Weston
FL
Declarações
SG declares that he has no competing interests.
Agradecimentos
Dr Franck F. Rahaghi and Dr Samuel Gurevich would like to gratefully acknowledge Dr Felix Hernandez, Dr Jose Gonzalez, and Dr Theodore W. Marcy, previous contributors to this topic.
Declarações
FH, JG, and TWM declare that they have no competing interests.
Revisores
Jaymin Morjaria, MBBS, FRCP, MD
Consultant Respiratory Physician
Guy’s and St Thomas NHS Foundation Trust
Harefield Hospital
Middlesex
Honorary Senior Lecturer
Brunel University
London
UK
Declarações
JM has received honoraria for speaking and financial support to attend meetings/advisory boards from Wyeth, Chiesi, Pfizer, MSD, Boehringer Ingelheim, Teva, GSK/Allen & Hanburys, Napp, Almirall, AstraZeneca, Trudell, Cook Medical, Medela AG, Medtronics, and Novartis. He has been an expert witness in a court case relating to the impact of smoking on illness severity, ITU admissions, and mortality from Covid-19 in South Africa in 2020.
Arran Woodhouse, MSc
Senior Tobacco Programme Manager
North Central London Integrated Care Board
London
UK
Declarações
AW has been reimbursed by Johnson & Johnson Ltd Nicorette UK Consulting for chairing the Smoking Cessation National Advisory Panel on 1 November 2022. He worked with NICE on the recent Tobacco Guideline (NG209) as a topic expert and as a specialist committee member for the treating dependence Quality Standards Advisory Committee (QS 207). He was appointed as an Expert Adviser for the NICE Centre for Guidelines in February 2023. Previous member of the British Thoracic Society's Tobacco Specialist Advisory Group.
Créditos aos pareceristas
Os tópicos do BMJ Best Practice são constantemente atualizados, seguindo os desenvolvimentos das evidências e das diretrizes. Os pareceristas aqui listados revisaram o conteúdo pelo menos uma vez durante a história do tópico.
Declarações
As afiliações e declarações dos pareceristas referem--se ao momento da revisão.
Referências
Principais artigos
United States Public Health Service Office of the Surgeon General; National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Smoking cessation: a report of the Surgeon General. 2020 [internet publication].Texto completo Resumo
Tobacco Use and Dependence Guideline Panel, US Department of Health and Human Services. Treating tobacco use and dependence: 2008 update. Rockville (MD): US Department of Health and Human Services; 2008.Texto completo
National Institute for Health and Care Excellence. Tobacco: preventing uptake, promoting quitting and treating dependence. Feb 2025 [internet publication].Texto completo
US Preventive Services Task Force. Interventions for tobacco smoking cessation in adults, including pregnant persons: US Preventive Services Task Force recommendation statement. JAMA. 2021 Jan 19;325(3):265-79.Texto completo Resumo
Barua RS, Rigotti NA, Benowitz NL, et al. 2018 ACC expert consensus decision pathway on tobacco cessation treatment: a report of the American College of Cardiology Task Force on clinical expert consensus documents. J Am Coll Cardiol. 2018 Dec 5;72(25):3332-65.Texto completo Resumo
Hartmann-Boyce J, Chepkin SC, Ye W, et al. Nicotine replacement therapy versus control for smoking cessation. Cochrane Database Syst Rev. 2018 May 31;(5):CD000146.Texto completo Resumo
Artigos de referência
Uma lista completa das fontes referenciadas neste tópico está disponível para os usuários com acesso total ao BMJ Best Practice.
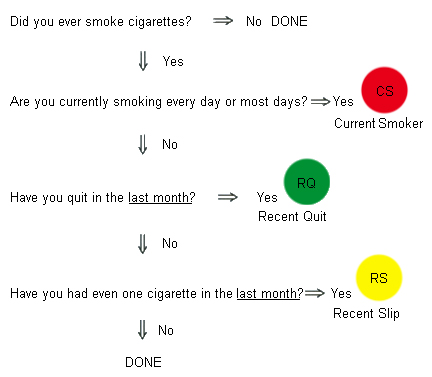
Diagnósticos diferenciais
- Substance use disorder
Mais Diagnósticos diferenciaisGuidelines
- NCCN clinical practice guidelines in oncology: smoking cessation
- Smoking and tobacco use: clinical cessation tools
Mais DiretrizesPatient information
Quitting smoking
More Patient informationLog in or subscribe to access all of BMJ Best Practice
Use of this content is subject to our disclaimer