Resumo
Definição
História e exame físico
Principais fatores diagnósticos
- blistering skin lesions
Outros fatores diagnósticos
- skin hyperpigmentation
- hypertrichosis
- scarring alopecia
- red urine
Fatores de risco
- male, middle-aged, white people
- alcohol use
- smoking
- estrogen therapy
- hepatitis C
- HIV
- hereditary hemochromatosis gene (HFE) mutation
- uroporphyrinogen decarboxylase (UROD) mutations
- exposure to halogenated polycyclic aromatic hydrocarbons
- reduced levels of antioxidants
- end-stage renal disease
- diabetes mellitus
Investigações diagnósticas
Primeiras investigações a serem solicitadas
- plasma total porphyrins
- plasma fluorescence emission
- urinary total porphyrins
- erythrocyte total porphyrins
Investigações a serem consideradas
- fractionation of plasma porphyrins by high-performance liquid chromatography (HPLC)
- fractionation of urinary porphyrins by HPLC
- erythrocyte uroporphyrinogen decarboxylase (UROD) activity
- fecal porphyrins
- DNA studies
- Liver function tests
- serum ferritin
- liver biopsy
- skin biopsy
- serum HIV enzyme-linked immunosorbent assay
- serum hepatitis C surface antibodies
- creatinine
- BUN
- hematocrit
- hemoglobin
Algoritmo de tratamento
no phlebotomy contraindications
phlebotomy contraindicated or poorly tolerated
relapse after remission
Colaboradores
Autores
Gagan Sood, MD
Associate Professor
Department of Medicine and Surgery
Baylor College of Medicine
Houston
TX
Declarações
GS is an author of a number of references cited in this topic.
Karl E. Anderson, MD
Professor
Departments of Preventive Medicine and Community Health and Internal Medicine
University of Texas Medical Branch
Galveston
TX
Declarações
KEA has received grants from the National Institutes of Health, the US Food and Drug Administration, and Alnylam Pharmaceuticals; he is an author of a number of references cited in this topic. KEA has received consulting fees, advisory board fees, and grants to the university from Alnylam Pharmaceuticals; consulting fees, advisory board fees, and grants from Recordati Rare Diseases; and consulting fees and grants from Mitsubishi Tanabe Pharma America.
Revisores
Robert S. Dawe, MBChB, MRCP(UK), MD(Glasgow)
Consultant Dermatologist
Honorary Clinical Senior Lecturer
Department of Dermatology
Ninewells Hospital & Medical School
Dundee
UK
Declarações
RSD declares that he has no competing interests.
Jeffrey P. Callen, MD
Professor of Medicine (Dermatology)
University of Louisville
Louisville
KY
გაფრთხილება:
JPC declares that he has no competing interests.
Montgomery Bissell, MD
Professor and Chief
Gastroenterology
University of California
San Francisco
CA
გაფრთხილება:
MB declares that he has no competing interests.
რეცენზენტების განცხადებები
BMJ Best Practice-ის თემების განახლება სხვადასხვა პერიოდულობით ხდება მტკიცებულებებისა და რეკომენდაციების განვითარების შესაბამისად. ქვემოთ ჩამოთვლილმა რეცენზენტებმა თემის არსებობის მანძილზე კონტენტს ერთხელ მაინც გადახედეს.
გაფრთხილება
რეცენზენტების აფილიაციები და გაფრთხილებები მოცემულია გადახედვის მომენტისთვის.
წყაროები
ძირითადი სტატიები
Phillips JD, Bergonia HA, Reilly CA, et al. A porphomethene inhibitor of uroporphyrinogen decarboxylase causes porphyria cutanea tarda. Proc Natl Acad Sci USA. 2007 Jan;104:5079-84.სრული ტექსტი აბსტრაქტი
Jalil S, Grady JJ, Lee C, et al. Associations among behavior-related susceptibility factors in porphyria cutanea tarda. Clin Gastroenterol Hepatol. 2010 Mar;8(3):297-302;e1.სრული ტექსტი აბსტრაქტი
Singal AK. Porphyria cutanea tarda: recent update. Mol Genet Metab. 2019 Nov;128(3):271-81. აბსტრაქტი
Handler NS, Handler MZ, Stephany MP, et al. Porphyria cutanea tarda: an intriguing genetic disease and marker. Int J Dermatol. 2017 Jun;56(6):e106-17. აბსტრაქტი
გამოყენებული სტატიები
ამ თემაში მოხსენიებული წყაროების სრული სია ხელმისაწვდომია მომხმარებლებისთვის, რომლებსაც აქვთ წვდომა BMJ Best Practice-ის ყველა ნაწილზე.
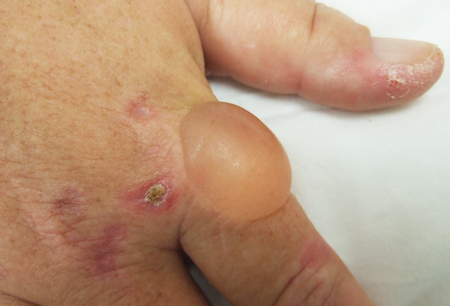
დიფერენციული დიაგნოზები
- Variegate porphyria (VP)
- Hereditary coproporphyria (HCP)
- Congenital erythropoietic porphyria (CEP)
მეტი დიფერენციული დიაგნოზებიშედით სისტემაში ან გამოიწერეთ BMJ Best Practice
ამ მასალის გამოყენება ექვემდებარება ჩვენს განცხადებას