Resumen
Definición
History and exam
Key diagnostic factors
- fever or history of fever
Other diagnostic factors
- headache
- weakness
- myalgia
- arthralgia
- anorexia
- diarrhea
- seizures
- nausea and vomiting
- abdominal pain
- pallor
- hepatosplenomegaly
- jaundice
- altered level of consciousness
- hypotension
- bleeding
- anuria/oliguria
- tachypnea
Risk factors
- travel to endemic area
- inadequate or absent chemoprophylaxis
- insecticide-treated bed net not used in endemic area
- low host immunity (severe disease)
- pregnancy (severe disease)
- age <5 years (severe disease)
- immunocompromise (severe disease)
- older age (severe disease)
- malnutrition (severe disease)
- iron administration (children)
Diagnostic investigations
1st investigations to order
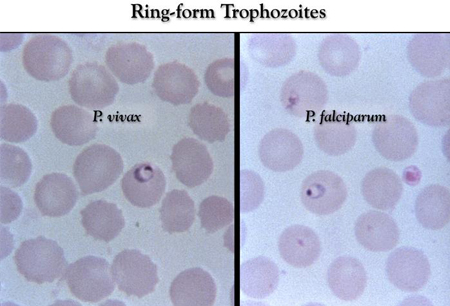
- Giemsa-stained thick and thin blood smears
- rapid diagnostic tests (RDTs)
- CBC
- clotting profile
- serum electrolytes, BUN and creatinine
- serum LFTs
- serum blood glucose
- urinalysis
- arterial blood gas
Investigations to consider
- polymerase chain reaction (PCR) blood for malaria
- chest x-ray
- blood culture
- urine culture
- sputum culture
- lumbar puncture
- HIV test
- PCR nasopharyngeal swabs for influenza or COVID-19
- CT head
Emerging tests
- loop-mediated isothermal amplification
Treatment algorithm
severe disease (or unable to take oral medication initially): all Plasmodium species
Plasmodium falciparum (or unknown species): uncomplicated disease
Plasmodium ovale: uncomplicated disease
Plasmodium vivax: uncomplicated disease
Plasmodium malariae or Plasmodium knowlesi: uncomplicated disease
Plasmodium falciparum: recurrent infection
Contributors
Authors
Elizabeth Ashley, MB BS, FRCP, FRCPath
Director
Institution Lao-Oxford-Mahosot Hospital - Wellcome Trust Research Unit
Vientiane
Laos
Honorary Consultant in Infectious Diseases and Microbiology
Oxford University Hospitals NHS Foundation Trust
Professor of Tropical Medicine
University of Oxford
Oxford
UK
Disclosures
EA is an associate editor of Malaria Journal, an academic editor for PLOS Medicine, and is on the Lancet Infectious Diseases International Advisory Board. EA is on the council of the International Society for Infectious Diseases. The Institution Lao-Oxford-Mahosot Hospital - Wellcome Trust Research Unit receives core funding from the Wellcome Trust. EA is an author of a number of references cited in this topic.
Arjun Chandna, BA MRCP AFHEA
Clinical Research Fellow
Centre for Tropical Medicine and Global Health
University of Oxford
Oxford
Specialty Registrar in Infectious Diseases and Medical Microbiology
University College London Hospitals NHS Trust
London
UK
Declarações
None.
Agradecimentos
Dr Elizabeth Ashley and Dr Arjun Chandna would like to gratefully acknowledge Professor Ron Behrens, Mariyam Mirfenderesky, Dr Signe Maj Sorensen, Dr Joanna Allen, Dr Simon Warren, and Dr Behzad Nadjm, previous contributors to this topic.
Declarações
RB acted as a paid expert to the courts on malaria prophylaxis. RB received fees on the Travel Health advisory board for Emergent BioSolutions. RB prepared education material for the Royal College of Physicians and Surgeons of Glasgow. RB is an author of a number of references cited in this topic. MM, SMS, JA, and SW declare that they have no competing interests. BN is an author of a reference cited in this topic.
Revisores
Blaise Genton, MD
Professor
Head of the Travel Clinic
Consultant of Tropical and Travel Medicine
University Hospital
Project Leader
Swiss Tropical and Public Health Institute
Basel
Switzerland
Declarações
BG has received a research grant from Novartis Pharma to assess the impact of the introduction of artemether-lumefantrine (Novartis) as first-line treatment for uncomplicated malaria on mortality of children under 5 years old in 2 districts in Tanzania and travel grants from Novartis Pharma to present the results of the study above. BG is an author of a reference cited in this topic.
David Sullivan, MD
Associate Professor
Malaria Research Institute and Department of Molecular Microbiology and Immunology
Johns Hopkins Bloomberg School of Public Health
Baltimore
MD
Declarações
DS has received royalties from antigen provision for a diagnostic test to Inverness. DS with Johns Hopkins University has patents on diagnostic tests that do not require blood.
Walther H. Wernsdorfer, MD
Professor
Institute of Specific Prophylaxis and Tropical Medicine
Medical University of Vienna
Vienna
Austria
Declarações
WHW declares that he has no competing interests.
Créditos aos pareceristas
Os tópicos do BMJ Best Practice são constantemente atualizados, seguindo os desenvolvimentos das evidências e das diretrizes. Os pareceristas aqui listados revisaram o conteúdo pelo menos uma vez durante a história do tópico.
Declarações
As afiliações e declarações dos pareceristas referem--se ao momento da revisão.
Referências
Principais artigos
World Health Organization. WHO guidelines for malaria. Nov 2024 [internet publication].Texto completo
Centers for Disease Control and Prevention. Clinical guidance: malaria diagnosis & treatment in the U.S. Jun 2024 [internet publication].Texto completo
Artigos de referência
Uma lista completa das fontes referenciadas neste tópico está disponível para os usuários com acesso total ao BMJ Best Practice.
Diagnósticos diferenciais
- Dengue fever
- Zika virus infection
- Chikungunya virus
Mais Diagnósticos diferenciaisDiretrizes
- WHO guidelines for malaria
- Malaria diagnosis and treatment in the US
Mais DiretrizesFolletos para el paciente
Malaria prevention
Más Folletos para el pacienteVideos
Diagnostic lumbar puncture in adults: animated demonstration
Más vídeosInicie sesión o suscríbase para acceder a todo el BMJ Best Practice
El uso de este contenido está sujeto a nuestra cláusula de exención de responsabilidad