Resumo
Definição
História e exame físico
Principais fatores diagnósticos
- visual disturbance in one eye
- peculiar sensory phenomena
Outros fatores diagnósticos
- female sex
- age 20-40 years
- foot dragging or slapping
- leg cramping
- fatigue
- urinary frequency
- bowel dysfunction
- spasticity/increased muscle tone
- increased deep tendon reflexes
- imbalance/incoordination
- pale optic disk or noncorrectable visual loss
- incorrect responses to Ishihara color blindness test plates
- abnormal eye movements
Fatores de risco
- female sex
- family history of MS
- northern latitude
- genetic factors
- smoking
- vitamin D deficiency
- autoimmune disease
- Epstein-Barr virus
- overweight/obesity in children and adolescents
Investigações diagnósticas
Primeiras investigações a serem solicitadas
- MRI - brain
- MRI - spinal cord
- CBC
- comprehensive metabolic panel
- thyroid-stimulating hormone
- vitamin B12
Investigações a serem consideradas
- antibody testing for neuromyelitis optica spectrum disorders (NMOSD)
- cerebrospinal fluid evaluation
- evoked potentials
Algoritmo de tratamento
acute relapse affecting function
relapsing-remitting MS
secondary progressive MS
primary progressive MS
Colaboradores
Autores
Mary Alissa Willis, MD
Associate Professor and Chair
Department of Neurology
University of Mississippi Medical Center
Jackson
MS
Declarações
MAW has been compensated for Speakers' Bureau participation with Alexion, Biogen, Bristol-Myers Squibb, Genzyme, and Genentech, and advisory board participation with Alexion, Biogen, Greenwich Biosciences, Genentech, and Horizon.
Agradecimentos
Dr Mary Alissa Willis would like to gratefully acknowledge Dr Lael A. Stone, a previous contributor to this topic. We have since been made aware that Dr Stone is deceased.
Revisores
Alex Rae-Grant, MD
Project Leader for Neurology
Neurological Institute
Cleveland Clinic
Cleveland
OH
Declarações
ARG declares that he has no competing interests.
Sarah A. Morrow, MD, FRCPC, MS
Associate Professor of Neurology
Department of Clinical Neurological Sciences
London Health Sciences Centre
University Hospital
Ontario
Canada
Declarações
SAM declares that she has no competing interests.
Marcelo Kremenchutzky, MD
Director
The London Multiple Sclerosis Clinic
Associate Professor
Schulich School of Medicine
University of Western Ontario
Neurologist
Clinical Neurological Sciences Department
University Hospital
London Health Sciences Centre
Ontario
Canada
Declarações
MK declares that he has no competing interests.
Abhijit Chaudhuri, DM, MD, PhD, FACP, FRCPGlasg, FRCPLond
Consultant Neurologist
Department of Neurology
Queen’s Hospital
Romford
UK
Declarações
AC declares that he has no competing interests.
Créditos aos pareceristas
Os tópicos do BMJ Best Practice são constantemente atualizados, seguindo os desenvolvimentos das evidências e das diretrizes. Os pareceristas aqui listados revisaram o conteúdo pelo menos uma vez durante a história do tópico.
Declarações
As afiliações e declarações dos pareceristas referem--se ao momento da revisão.
Referências
Principais artigos
Wattjes MP, Ciccarelli O, Reich DS, et al. 2021 MAGNIMS-CMSC-NAIMS consensus recommendations on the use of MRI in patients with multiple sclerosis. Lancet Neurol. 2021 Aug;20(8):653-670.Texto completo Resumo
Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018 Feb;17(2):162-73. Resumo
Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler. 2018 Feb;24(2):96-120.Texto completo Resumo
Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis. Neurology. 2018 Apr 24;90(17):777-88.Texto completo Resumo
Solari A, Giordano A, Sastre-Garriga J, et al. EAN guideline on palliative care of people with severe, progressive multiple sclerosis. Eur J Neurol. 2020 Aug;27(8):1510-29.Texto completo Resumo
Artigos de referência
Uma lista completa das fontes referenciadas neste tópico está disponível para os usuários com acesso total ao BMJ Best Practice.
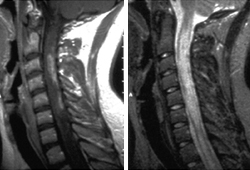
Diagnósticos diferenciais
- Myelopathy due to cervical spondylosis
- Fibromyalgia
- Postural orthostatic tachycardia syndrome with or without cervicogenic migraine
Mais Diagnósticos diferenciaisDiretrizes
- Clinical guidance in neuropalliative care: an AAN position statement
- Multiple sclerosis in adults: management
Mais DiretrizesFolhetos informativos para os pacientes
Multiple sclerosis
Multiple sclerosis: questions to ask your doctor
Mais Folhetos informativos para os pacientesVideos
Diagnostic lumbar puncture in adults: animated demonstration
Venepuncture and phlebotomy: animated demonstration
Mais vídeosConectar-se ou assinar para acessar todo o BMJ Best Practice
O uso deste conteúdo está sujeito ao nosso aviso legal