Resumo
Definição
História e exame físico
Principais fatores diagnósticos
- men ages >40 years
- use of gout-inducing medication
- consumption of meat, seafood, or alcohol
- history of medical condition with high cell turnover rate
- rapid-onset severe pain
- joint stiffness
- foot joint distribution
- few affected joints
- swelling and joint effusion
- tenderness
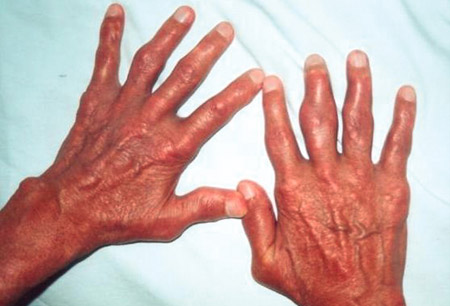
- tophi
Outros fatores diagnósticos
- erythema and warmth
- family history of gout
Fatores de risco
- older age
- male sex
- menopausal status
- consumption of meat, seafood, alcohol
- use of diuretics
- use of cyclosporine or tacrolimus
- use of pyrazinamide
- use of aspirin
- genetic susceptibility
- high cell turnover rate
- obesity
- adiposity and insulin resistance
- exogenous insulin
- hypertension
- renal insufficiency
- diabetes mellitus
- hyperlipidemia
- family history of gout
Diagnostic tests
1st tests to order
- arthrocentesis with synovial fluid analysis
Tests to consider
- serum uric acid level
- ultrasound
- dual energy computed tomography (DECT)
- x-ray of affected joint
Treatment algorithm
acute gout
recurrent gout: 2-3 weeks post acute episode
Contributors
Authors
Fadi Badlissi, MD, MSc

Assistant Professor
Harvard Medical School
Attending Physician
Director of the Musculoskeletal Medicine Unit
Department of Orthopedics & Division of Rheumatology
Beth Israel Deaconess Medical Center
Boston
MA
Disclosures
FB has received an honorarium as an advisory board member for Horizon Pharmaceuticals.
Peer reviewers
H. Ralph Schumacher, Jr., MD
Professor of Medicine
VA Medical Center
Philadelphia
PA
Disclosures
HRS has been a consultant for a number of pharmaceutical companies that produce drugs that can be used for the treatment of gout. Some companies have supplied HRS with funding. HRS is an author of a number of references cited in this topic.
Ade Adebajo, MD
Associate Director of Teaching and Honorary Senior Lecturer in Rheumatology
Academic Rheumatology Group
Faculty of Medicine
University of Sheffield
Sheffield
UK
Disclosures
AA declares that he has no competing interests.
Martin Underwood, MBBS
Professor of Primary Care Research
Warwick Medical School
Coventry
UK
Disclosures
MU declares that he has no competing interests.
Peer reviewer acknowledgements
BMJ Best Practice topics are updated on a rolling basis in line with developments in evidence and guidance. The peer reviewers listed here have reviewed the content at least once during the history of the topic.
Disclosures
Peer reviewer affiliations and disclosures pertain to the time of the review.
References
Key articles
National Institute for Health and Care Excellence. Gout: diagnosis and management. June 2022. [internet publication].Full text
FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020 Jun;72(6):744-60. Abstract
Qaseem A, Harris RP, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Management of acute and recurrent gout: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017 Jan 3;166(1):58-68.Full text Abstract
Richette P, Doherty M, Pascual E, et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis. 2017 Jan;76(1):29-42.Full text Abstract
Reference articles
A full list of sources referenced in this topic is available to users with access to all of BMJ Best Practice.
Differentials
- Pseudogout (calcium pyrophosphate deposition disease)
- Septic arthritis
- Trauma
More DifferentialsGuidelines
- Gout: diagnosis and management
- Guideline for the management of gout
More GuidelinesPatient information
Gout
More Patient informationVideos
Aspiration and injection of the knee: animated demonstration
Aspiration and injection of the shoulder animated demonstration
More videosLog in or subscribe to access all of BMJ Best Practice
Use of this content is subject to our disclaimer