Summary
Definition
History and exam
Key diagnostic factors
- cough
- unpleasant sensation in throat
- postnasal drip
- cobblestone mucosa
Other diagnostic factors
- nasal abnormalities
- symptoms of rhinitis
- posterior pharyngeal mucus
- wheeze
- voice disturbance
- heartburn/indigestion
Risk factors
- seasonal triggers
- occupational triggers
- female gender
Diagnostic tests
1st tests to order
- empiric treatment trial
- chest x-ray
- CBC
- spirometry
- fractional exhaled nitric oxide
Tests to consider
- direct nasolaryngoscopy
- serum IgE level
- specific aeroallergen radioallergosorbent test (RAST)
- peak expiratory flow (PEF)
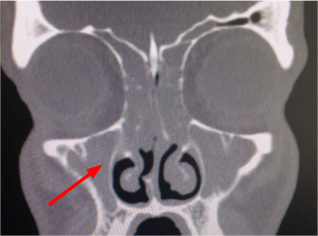
- CT sinus
- CT chest with or without intravenous contrast
Emerging tests
- bronchial challenge test
Treatment algorithm
suspected upper airway cough syndrome (UACS)
confirmed upper airway cough syndrome (UACS)
Contributors
Authors
Kian Fan Chung, MD, FRCP
Professor of Respiratory Medicine
Head of Experimental Studies Medicine
National Heart and Lung Institute
Imperial College London
Royal Brompton Hospital
London
UK
Disclosures
KFC has received honoraria for participating in advisory board meetings on asthma, chronic obstructive pulmonary disease and chronic cough for AstraZeneca, Boehringer Ingelheim, GSK, Merck, Nocion, Novartis, Roche, and Shionogi, and has received remuneration for speaking engagements for AstraZeneca, GSK, Novartis, and Sanofi. KFC has organised the 13th London International Cough Symposium in July 2024 at Imperial College, which was supported by educational grants from Astra-Zeneca, GSK, NeRRe, Nocion, Reckitt, Sensory Cloud, Siva, and Trevi. KFC is an investigator on research supported by research grants to KFC’s institution from GSK, Merck and UK Research and Innovation. KFC has received remuneration for developing educational material on chronic cough for EPG Health.
Woo-Jung Song, MD, PhD
Professor
Department of Allergy and Clinical Immunology
Asian Medical Center
University of Ulsan College of Medicine
Seoul
Republic of Korea
Disclosures
WJS declares grants from Merck Sharp & Dohme Corp. and AstraZeneca, consulting fees from Merck, Bellus, AstraZeneca, Shionogi, and GSK, and lecture fees from Merck, AstraZeneca, GSK, Sanofi, and Novartis.
Acknowledgements
Professor Kian Fan Chung and Professor Woo-Jung Song would like to gratefully acknowledge Dr James H. Hull, a previous contributor to this topic.
Disclosures
JHH has received advisory board fees from Menlo Pharmaceuticals, and speaking and travel fees from TEVA and Boehringer Ingelheim.
Peer reviewers
J. Mark Madison, MD
Professor of Medicine
Chief
Pulmonary, Allergy and Critical Care Medicine
University of Massachusetts Medical School
Worcester
MA
Disclosures
JMM declares that he has no competing interests.
Omar S. Usmani, MBBS, PhD, FHEA, FRCP
NIHR Career Development Fellow
Clinical Senior Lecturer
Imperial College London
Consultant Physician in Respiratory & Internal Medicine
Royal Brompton Hospital
London
UK
Disclosures
OSU declares that he has no competing interests.
Jing Gennie Wang, MD
Assistant Professor
The Ohio State University
Columbus
OH
Disclosures
JW declares no competing interests.
Peer reviewer acknowledgements
BMJ Best Practice topics are updated on a rolling basis in line with developments in evidence and guidance. The peer reviewers listed here have reviewed the content at least once during the history of the topic.
Disclosures
Peer reviewer affiliations and disclosures pertain to the time of the review.
References
Key articles
Pratter M. Chronic upper airway cough syndrome secondary to rhinosinus diseases (previously referred to as postnasal drip syndrome): ACCP evidence-based clinical practice guidelines. Chest. 2006;129(suppl 1):63S-71S.Full text Abstract
European Respiratory Society. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. 2020 [internet publication].Full text
Gibson P, Wang G, McGarvey L, et al. Treatment of unexplained chronic cough: CHEST guideline and expert panel report. Chest. 2016;149:27-44.Full text Abstract
Reference articles
A full list of sources referenced in this topic is available to users with access to all of BMJ Best Practice.
Diagnósticos diferenciais
- Asthma
- Bronchiectasis
- Gastroesophageal reflux disease
Mais Diagnósticos diferenciaisDiretrizes
- Cough in children and adults: diagnosis, assessment and management (CICADA). Summary of an updated position statement on chronic cough in Australia
- British Thoracic Society Clinical Statement on chronic cough in adults
Mais DiretrizesConectar-se ou assinar para acessar todo o BMJ Best Practice
O uso deste conteúdo está sujeito ao nosso aviso legal