Summary
Definition
History and exam
Key diagnostic factors
- exposure to RSV
- infants at high risk for RSV infection
- winter season
- older adult age
- immune deficiency
- rhinorrhea/congestion
- tachypnea
- increased work of breathing
- cough
- wheeze
- poor feeding
- cyanosis
- rales
- apnea
Other diagnostic factors
- fever
Risk factors
- exposure to RSV
- hemodynamically significant congenital heart disease
- history of prematurity
- immune deficiency
- chronic lung disease
- indigenous/American-Indians/Alaska native infants and young children
- infants aged <6 months
- winter season
- older adult age
- smoke exposure
- family history of asthma
- Down syndrome
Diagnostic tests
1st tests to order
- pulse oximetry
Tests to avoid
- broad respiratory pathogen panels
Tests to consider
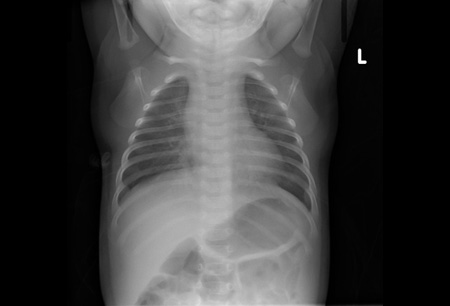
- chest x-ray
- hydration status
- rapid antigen detection from respiratory specimen (e.g., nasopharyngeal aspirate)
- reverse transcriptase polymerase chain reaction of respiratory specimen (e.g., nasopharyngeal aspirate)
Treatment algorithm
mild or self-limited illness
moderate illness
severe illness
Contributors
Authors
Giovanni Piedimonte, MD, FAAP, FCCP
Vice President for Research
Professor of Pediatrics, Biochemistry & Molecular Biology
Tulane University School of Medicine
New Orleans
LA
Disclosures
GP declares that he has no competing interests.
Margot Anderson, MD
Assistant Professor of Clinical Pediatrics
Section of Infectious Diseases and Hospital Medicine
Tulane University School of Medicine
Tulane University
New Orleans
LA
Disclosures
MA declares that she has no competing interests.
Acknowledgements
Dr Giovanni Piedimonte and Dr Margot Anderson would like to gratefully acknowledge Dr Frank Esper and Dr Melvin L. Wright, previous contributors to this topic.
Disclosures
FE is on an advisory board for Procter and Gamble. MLW declares that he has no competing interests.
Peer reviewers
Leonard R. Krilov, MD
Chief
Pediatric Infectious Disease
Vice Chairman
Department of Pediatrics
Children's Medical Center
Winthrop University Medical Center
Mineola
Professor of Pediatrics
School of Medicine
Stony Brook University Medical Center
Stony Brook
NY
Disclosures
LRK has participated as an investigator in multiple clinical research trials supported by grants from MedImmune. LRK has also served as a consultant to MedImmune on medical advisory boards and is a member of their speakers' bureau.
Robert Welliver, MD
Professor of Pediatrics
Women and Children's Hospital
Buffalo
NY
Disclosures
RW declares that he has no competing interests.
Jennifer Handforth, MB ChB, MRCPCH, DTM&H
Consultant Paediatrician
Croydon University Hospital
Croydon
UK
Disclosures
JH declares that she has no competing interests.
Peer reviewer acknowledgements
BMJ Best Practice topics are updated on a rolling basis in line with developments in evidence and guidance. The peer reviewers listed here have reviewed the content at least once during the history of the topic.
Disclosures
Peer reviewer affiliations and disclosures pertain to the time of the review.
References
Key articles
Bont L, Checchia PA, Fauroux B, et al. Defining the epidemiology and burden of severe respiratory syncytial virus infection among infants and children in western countries. Infect Dis Ther. 2016 Sep;5(3):271-98.Full text Abstract
Ralston SL, Lieberthal AS, Meissner HC, et al; American Academy of Pediatrics. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014 Nov;134(5):e1474-502.Full text Abstract
Committee on Infectious Diseases; American Academy of Pediatrics. Red book. 32nd ed. Elk Grove Village, IL: AAP; 2021.Full text
Reference articles
A full list of sources referenced in this topic is available to users with access to all of BMJ Best Practice.
Differentials
- Human metapneumovirus
- Influenza virus
- Parainfluenza virus
More DifferentialsGuidelines
- Australasian bronchiolitis guideline
- Bronchiolitis in children: diagnosis and management
More GuidelinesPatient information
Bronchiolitis
Asthma in children
More Patient informationLog in or subscribe to access all of BMJ Best Practice
Use of this content is subject to our disclaimer