小结
定义
病史和体格检查
关键诊断因素
- positive family history
- presence of an associated syndrome
- decreased peripheral vision
- night blindness
- impaired dark adaptation
- decreased central acuity
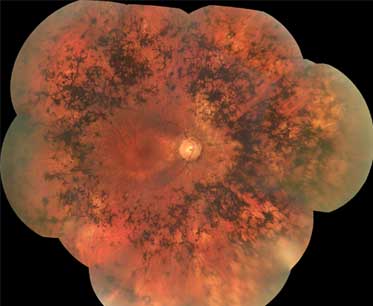
- atrophy of retinal pigment epithelium
- bone spicule pigmentation
其他诊断因素
- waxy pale optic nerve
- photopsias
- refractive error
- cataracts
- retinal vascular attenuation
- cystoid macular edema
- vitreous cells
- glare from bright lights
- abnormal color vision
- keratoconus
- glaucoma
- optic nerve head drusen
- Coats-like retinopathy
- Leber congenital amaurosis
危险因素
- family history
- presence of an associated syndrome
诊断性检查
首要检查
- assessment of visual acuity
- anterior segment exam and intraocular pressure measurement
- full field perimetry
- full field electroretinogram (ERG)
需考虑的检查
- elevated final dark-adapted threshold
- optical coherence tomography (OCT)
- genetic testing
- adaptive optics imaging
- wide-field fundus autofluorescence (FAF)
新兴检查
- whole exome sequencing
治疗流程
all patients
撰稿人
作者
Lesley Everett , MD, PhD, MPhil
Assistant Professor of Ophthalmology
Casey Eye Institute
Oregon Health and Sciences University
Divisions of Ophthalmic Genetics and Retina
Portland
OR
Declarações
LAE is supported by a Foundation Fighting Blindness Career Development Grant.
Mark E. Pennesi, MD, PhD

Professor
Casey Eye Institute
Oregon Health and Sciences University
Portland
OR
Declarações
MEP serves on the scientific advisory board and executive committee for the Foundation Fighting Blindness.
Paul Yang, MD, PhD

Associate Professor
Casey Eye Institute
Oregon Health and Sciences University
Portland
OR
Declarações
PY acted as a consultant for 4D Molecular Therapeutics, AAVantgarde Bio (IDMC), Adverum, Astellas, Beacon Therapeutics, BlueRock Therapeutics, Eluminex Biosciences, Foundation Fighting Blindness (SAB), Janssen (DSMB), MieraGTx (DSMB), Nanoscope Therapeutics (SAB), and TeamedOn.
Agradecimentos
Dr Lesley Everett, Dr Mark E. Pennesi, and Dr Paul Yang would like to gratefully acknowledge Dr Richard G. Weleber and Dr Peter J. Francis, previous contributors to this topic.
Declarações
RGW has served as a consultant to Novartis, Pfizer, and Wellstat, is a member of the scientific advisory board for Applied Genetic Technologies Corp, and serves on the scientific advisory board for the Foundation Fighting Blindness (the relationship has been reviewed and managed by Oregon Health & Science University). RGW also reports having received grants and personal fees from the Foundation Fighting Blindness and Applied Genetic Technologies Corp, and other support from Sanofi-Fovea, all outside the submitted work. In addition, RGW has a patent (US patent 8,657,446, Method and apparatus for visual field monitoring, also known as Visual Field Monitoring and Analysis, or VFMA, which has not been issued). PJF declares that he has no competing interests.
Revisores
Scott Fraser, MD, FRCS (Ed), FRCOphth
Consultant Ophthalmologist
Sunderland Eye Infirmary
Sunderland
UK
Declarações
SF declares that he has no competing interests.
Elias Traboulsi, MD
Professor of Ophthalmology
Director
Center for Genetic Eye Diseases
Cole Eye Institute
Cleveland Clinic
Cleveland
OH
Declarações
ET declares that he has no competing interests.
Créditos aos pareceristas
Os tópicos do BMJ Best Practice são constantemente atualizados, seguindo os desenvolvimentos das evidências e das diretrizes. Os pareceristas aqui listados revisaram o conteúdo pelo menos uma vez durante a história do tópico.
Declarações
As afiliações e declarações dos pareceristas referem--se ao momento da revisão.
Referências
Principais artigos
American Academy of Ophthalmology. Comprehensive adult medical eye evaluation PPP. Nov 2020 [internet publication].Texto completo
American Academy of Ophthalmology. Guidelines on clinical assessment of patients with inherited retinal degenerations - 2022. Oct 2022 [internet publication].Texto completo
Robson AG, Frishman LJ, Grigg J, et al. ISCEV Standard for full-field clinical electroretinography (2022 update). Doc Ophthalmol. 2022 Jun;144(3):165-77.Texto completo Resumo
American Academy of Ophthalmology. Recommendations for genetic testing of inherited eye diseases. February 2014 [internet publication].Texto completo
Artigos de referência
Uma lista completa das fontes referenciadas neste tópico está disponível para os usuários com acesso total ao BMJ Best Practice.
Diagnósticos diferenciais
- Congenital rubella
- Syphilis
- Vitamin A deficiency
Mais Diagnósticos diferenciaisDiretrizes
- Guidelines on clinical assessment of patients with inherited retinal degenerations
- Pediatric eye evaluations preferred practice pattern
Mais DiretrizesConectar-se ou assinar para acessar todo o BMJ Best Practice
O uso deste conteúdo está sujeito ao nosso aviso legal