Summary
Definition
History and exam
Key diagnostic factors
- skin lesions
Other diagnostic factors
- family history
- joint swelling or pain
Risk factors
- genetic
- infection
- local trauma
- medications
- stress
- smoking
- ethnicity
- alcohol
- greater body mass index (BMI)
Diagnostic tests
1st tests to order
- clinical diagnosis
Tests to consider
- skin biopsy
Treatment algorithm
plaque
guttate
pustular
Contributors
Authors
Andrea Chiricozzi, MD
Assistant Professor
University of Pisa
Consultant Dermatologist
University Hospital of Pisa
Pisa
Italy
Disclosures
AC has served as an advisory board member and consultant and has received fees and speaker's honoraria or has participated in clinical trials for AbbVie, Almirall, Biogen, Fresenius Kabi, Leo Pharma, Lilly, Janssen, Novartis, Sanofi Genzyme, and UCB Pharma; AC is also an author of a reference cited in this topic.
Acknowledgements
Dr Andrea Chiricozzi would like to gratefully acknowledge Dr April W. Armstrong and Dr Tsu-Yi Chuang, previous contributors to this topic.
Disclosures
TC declares that he has no competing interests. AWA is an investigator and consultant for Abbott, Amgen, and Janssen; AWA is also an author of references cited in this topic.
Peer reviewers
David Burden, MD, FRCP
Western Infirmary, Glasgow
Consultant Dermatologist Dermatology
Glasgow
UK
Disclosures
DB has been reimbursed as a consultant, researcher, and lecturer for Abbott, Leo, Pfizer, Merck, Janssen-Cilag, and Novartis.
Paradi Mirmirani, MD
Physician
Department of Dermatology
Kaiser Permanente Vallejo Medical Center
Vallejo
CA
Disclosures
PM declares that she has no competing interests.
Peer reviewer acknowledgements
BMJ Best Practice topics are updated on a rolling basis in line with developments in evidence and guidance. The peer reviewers listed here have reviewed the content at least once during the history of the topic.
Disclosures
Peer reviewer affiliations and disclosures pertain to the time of the review.
References
Key articles
Smith CH, Yiu ZZN, Bale T, et al. British Association of Dermatologists guidelines for biologic therapy for psoriasis 2020: a rapid update. Br J Dermatol. 2020 Oct;183(4):628-37.Full text Abstract
Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology – National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019 Sep;81(3):775-804.Full text Abstract
Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology - National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020 Jun;82(6):1445-86.Full text Abstract
National Institute for Health and Care Excellence. Psoriasis: assessment and management. September 2017 [internet publication].Full text Abstract
Reference articles
A full list of sources referenced in this topic is available to users with access to all of BMJ Best Practice.
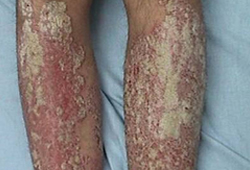
Differentials
- Eczema
- Pityriasis rosea
- Seborrheic dermatitis
More DifferentialsGuidelines
- Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures
- EuroGuiDerm guideline on the systemic treatment of psoriasis vulgaris – part 2: specific clinical and comorbid situations
More GuidelinesPatient information
Psoriasis: what is it?
Psoriasis: what are the treatment options?
More Patient informationCalculators
PASI Psoriasis Area and Severity Index
More CalculatorsLog in or subscribe to access all of BMJ Best Practice
Use of this content is subject to our disclaimer