Summary
Definition
ანამნეზი და გასინჯვა
ძირითადი დიაგნოსტიკური ფაქტორები
- rectal bleeding
- diarrhea
- blood in stool
სხვა დიაგნოსტიკური ფაქტორები
- abdominal pain
- arthritis and spondylitis
- malnutrition
- abdominal tenderness
- fever
- weight loss
- constipation
- skin rash
- uveitis and episcleritis
- pallor
რისკფაქტორები
- family history of inflammatory bowel disease
- human leukocyte antigen-B27
- infection
- use of nonsteroidal anti-inflammatory drugs (NSAIDs)
- not smoking or former smoker
დიაგნოსტიკური კვლევები
1-ად შესაკვეთი გამოკვლევები
- stool studies for infective pathogens
- fecal calprotectin (FC)
- CBC
- comprehensive metabolic panel (including LFTs)
- erythrocyte sedimentation rate (ESR)
- CRP
- plain abdominal radiograph
- flexible sigmoidoscopy
- colonoscopy
- biopsies
Tests to avoid
- serologic antibody testing
გასათვალისწინებელი კვლევები
- CT scan
- intestinal ultrasound
მკურნალობის ალგორითმი
acute severe ulcerative colitis (UC)
moderate-to-severe disease
mild disease
disease in remission
კონტრიბუტორები
ავტორები
Andrew Poullis, BSc, MBBS, MD, FRCP
Consultant Gastroenterologist
Department of Gastroenterology
St George’s Hospital
London
UK
გაფრთხილება:
AP is an author of a reference cited in this topic.
Sailish Honap, MBChB (Hons), MRes, MRCP (UK)
Consultant Gastroenterologist
Department of Gastroenterology
St George’s Hospital
London
UK
გაფრთხილება:
SH has received honoraria from Pfizer (speaker, consultant, advisory board member, and received travel grants), Janssen (speaker fees, travel grants), AbbVie (speaker fees, consultant, travel grants), Takeda (speaker fees), Ferring (travel grants), Pharmacosmos (travel grants), Galapagos (travel grants).
Kamal Patel,
გაფრთხილება:
all disclosures
Aaron Bhakta, BMedSci (Hons), BMBS, MSc, MRCP
Clinical Fellow in Gastroenterology
Department of Gastroenterology
St George’s Hospital
London
UK
გაფრთხილება:
AB declares that he has no competing interests.
მადლიერება
Dr Hesam A. Nooredinvand, and Dr Andrew Poullis would like to gratefully acknowledge Dr Ben Shandro, Dr Anet Soubieres, Dr Rhys Hewett, Dr Willem J.S. de Villiers, and Dr Houssam Mardini, previous contributors to this topic.
გაფრთხილება:
BS, AS, RH, WJSD, and HM declare that they have no competing interests.
რეცენზენტები
Stephen B Hanauer, MD
Professor of Medicine
Gastroenterology and Hepatology
Northwestern University
Evanston
IL
გაფრთხილება:
SW declares that he has no competing interests.
რეცენზენტების განცხადებები
BMJ Best Practice-ის თემების განახლება სხვადასხვა პერიოდულობით ხდება მტკიცებულებებისა და რეკომენდაციების განვითარების შესაბამისად. ქვემოთ ჩამოთვლილმა რეცენზენტებმა თემის არსებობის მანძილზე კონტენტს ერთხელ მაინც გადახედეს.
გაფრთხილება
რეცენზენტების აფილიაციები და გაფრთხილებები მოცემულია გადახედვის მომენტისთვის.
წყაროები
ძირითადი სტატიები
World Gastroenterology Organisation. Global guidelines: inflammatory bowel disease. Aug 2015 [internet publication].სრული ტექსტი
Rubin DT, Ananthakrishnan AN, Siegel CA, et al. ACG clinical guideline update: ulcerative colitis in adults. Am J Gastroenterol. 2025 Jun 3;120(6):1187-224.სრული ტექსტი აბსტრაქტი
Moran GW, Gordon M, Sinopolou V, et al. British Society of Gastroenterology guidelines on inflammatory bowel disease in adults: 2025. Gut. 2025 Jun 23;74(Suppl 2):s1-101.სრული ტექსტი აბსტრაქტი
Singh S, Loftus EV Jr, Limketkai BN, et al. AGA living clinical practice guideline on pharmacological management of moderate-to-severe ulcerative colitis. Gastroenterology. 2024 Dec;167(7):1307-43.სრული ტექსტი აბსტრაქტი
Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. 2017 Jul 1;11(7):769-84.სრული ტექსტი აბსტრაქტი
National Institute for Health and Care Excellence (UK). Ulcerative colitis: management. May 2019 [internet publication].სრული ტექსტი
გამოყენებული სტატიები
ამ თემაში მოხსენიებული წყაროების სრული სია ხელმისაწვდომია მომხმარებლებისთვის, რომლებსაც აქვთ წვდომა BMJ Best Practice-ის ყველა ნაწილზე.
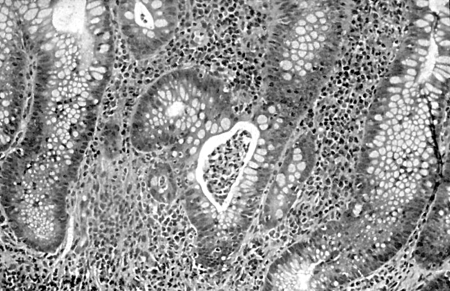
დიფერენციული დიაგნოზები
- Crohn disease
- Indeterminate colitis
- Radiation colitis
მეტი დიფერენციული დიაგნოზებიGuías de práctica clínica
- Preventive care in inflammatory bowel disease
- Management of inflammatory bowel disease in adults
მეტი Guías de práctica clínicaFolletos para el paciente
Ulcerative colitis: what is it?
Ulcerative colitis: what are the treatment options?
Más Folletos para el pacienteVideos
Venepuncture and phlebotomy: animated demonstration
Más vídeosInicie sesión o suscríbase para acceder a todo el BMJ Best Practice
El uso de este contenido está sujeto a nuestra cláusula de exención de responsabilidad