Summary
Definition
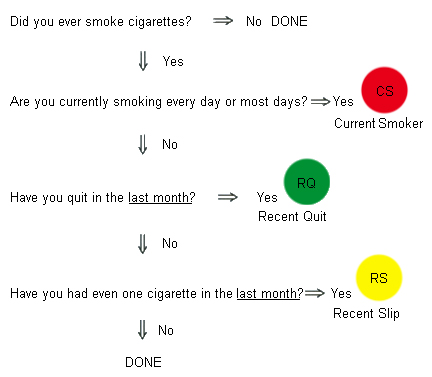
History and exam
Key diagnostic factors
- number of cigarettes per day
- time to first cigarette (TTFC)
- use of alternative tobacco and nicotine delivery products
- history of substance use disorder (SUD)
- pregnancy or breastfeeding
Other diagnostic factors
- history of depression
- history of schizophrenia
- seizure disorder
- hypertension
- unstable cardiac disease
- ventricular arrhythmia
- asthma
- chronic obstructive pulmonary disease (COPD)
- temporomandibular joint or dental disorder
Risk factors
- age <24 years
- low socioeconomic status
- history of mental illness or substance use disorder
- history of HIV/AIDS
- use of alternative tobacco and nicotine delivery products
- genetics
Diagnostic tests
1st tests to order
- self-report of smoking status
- Fagerström Test For Nicotine Dependence (FTND)
- Heaviness of Smoking Index (HSI)
Tests to consider
- carbon monoxide (CO) monitoring
- cotinine level
Treatment algorithm
hospitalized active smokers
active smokers ready to quit: adults (not pregnant/breastfeeding)
active smokers ready to quit: pregnant/breastfeeding women or adolescents
active smokers not ready to quit
Contributors
Authors
Franck F. Rahaghi, MD, MHS, FCCP
Chairman
Pulmonary Department
Director
Pulmonary Education and Rehabilitation
Cleveland Clinic
Weston
FL
Disclosures
FFR is a consultant and speaker for Bayer, United Therapeutics, Janssen-PH, Merck, Boehringer Ingleheim, Takeda, and Talecris. He is involved in industry-sponsored research with Bayer, Janssen-PH, Gossamer Bio, and Bellerophon.
Samuel Gurevich, MD, FCCP
Pulmonary Department
Director
Respiratory Therapy
Cleveland Clinic
Weston
FL
Disclosures
SG declares that he has no competing interests.
Acknowledgements
Dr Franck F. Rahaghi and Dr Samuel Gurevich would like to gratefully acknowledge Dr Felix Hernandez, Dr Jose Gonzalez, and Dr Theodore W. Marcy, previous contributors to this topic.
Disclosures
FH, JG, and TWM declare that they have no competing interests.
Peer reviewers
Jaymin Morjaria, MBBS, FRCP, MD
Consultant Respiratory Physician
Guy’s and St Thomas NHS Foundation Trust
Harefield Hospital
Middlesex
Honorary Senior Lecturer
Brunel University
London
UK
Disclosures
JM has received honoraria for speaking and financial support to attend meetings/advisory boards from Wyeth, Chiesi, Pfizer, MSD, Boehringer Ingelheim, Teva, GSK/Allen & Hanburys, Napp, Almirall, AstraZeneca, Trudell, Cook Medical, Medela AG, Medtronics, and Novartis. He has been an expert witness in a court case relating to the impact of smoking on illness severity, ITU admissions, and mortality from Covid-19 in South Africa in 2020.
Arran Woodhouse, MSc
Senior Tobacco Programme Manager
North Central London Integrated Care Board
London
UK
Disclosures
AW has been reimbursed by Johnson & Johnson Ltd Nicorette UK Consulting for chairing the Smoking Cessation National Advisory Panel on 1 November 2022. He worked with NICE on the recent Tobacco Guideline (NG209) as a topic expert and as a specialist committee member for the treating dependence Quality Standards Advisory Committee (QS 207). He was appointed as an Expert Adviser for the NICE Centre for Guidelines in February 2023. Previous member of the British Thoracic Society's Tobacco Specialist Advisory Group.
Peer reviewer acknowledgements
BMJ Best Practice topics are updated on a rolling basis in line with developments in evidence and guidance. The peer reviewers listed here have reviewed the content at least once during the history of the topic.
Disclosures
Peer reviewer affiliations and disclosures pertain to the time of the review.
References
Key articles
United States Public Health Service Office of the Surgeon General; National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Smoking cessation: a report of the Surgeon General. 2020 [internet publication].Full text Abstract
Tobacco Use and Dependence Guideline Panel, US Department of Health and Human Services. Treating tobacco use and dependence: 2008 update. Rockville (MD): US Department of Health and Human Services; 2008.Full text
National Institute for Health and Care Excellence. Tobacco: preventing uptake, promoting quitting and treating dependence. Feb 2025 [internet publication].Full text
US Preventive Services Task Force. Interventions for tobacco smoking cessation in adults, including pregnant persons: US Preventive Services Task Force recommendation statement. JAMA. 2021 Jan 19;325(3):265-79.Full text Abstract
Barua RS, Rigotti NA, Benowitz NL, et al. 2018 ACC expert consensus decision pathway on tobacco cessation treatment: a report of the American College of Cardiology Task Force on clinical expert consensus documents. J Am Coll Cardiol. 2018 Dec 5;72(25):3332-65.Full text Abstract
Hartmann-Boyce J, Chepkin SC, Ye W, et al. Nicotine replacement therapy versus control for smoking cessation. Cochrane Database Syst Rev. 2018 May 31;(5):CD000146.Full text Abstract
Reference articles
A full list of sources referenced in this topic is available to users with access to all of BMJ Best Practice.
Differentials
- Substance use disorder
More DifferentialsGuidelines
- NCCN clinical practice guidelines in oncology: smoking cessation
- Smoking and tobacco use: clinical cessation tools
More GuidelinesPatient information
Quitting smoking
More Patient informationLog in or subscribe to access all of BMJ Best Practice
Use of this content is subject to our disclaimer