Summary
Definition
History and exam
Key diagnostic factors
- polyuria
- polydipsia
- diabetic ketoacidosis
Other diagnostic factors
- young age
- weight loss
- blurred vision
- nausea and vomiting
- abdominal pain
- tachypnea
- clinical dehydration
- lethargy
- altered mental status
Risk factors
- genetic predisposition
- geographic region
- infectious agents
- dietary factors
- use of immunotherapy
დიაგნოსტიკური კვლევები
1-ად შესაკვეთი გამოკვლევები
- HbA1c
- fasting plasma glucose
- 2-hour post-glucose load plasma glucose
- random plasma glucose
გასათვალისწინებელი კვლევები
- plasma or urine ketones
- random C-peptide
- autoimmune markers
მკურნალობის ალგორითმი
nonpregnant
pregnant
კონტრიბუტორები
ავტორები
Rajesh K. Garg, MD

Professor of Medicine
David Geffen School of Medicine at UCLA
Harbor-UCLA Medical Center
Torrance
CA
გაფრთხილება:
RKG is an author of a number of references cited in this topic.
მადლიერება
Dr Rajesh K. Garg would like to gratefully acknowledge Dr Varsha Vimalananda, previous contributor to this topic.
გაფრთხილება:
VV declares that she has no competing interests.
რეცენზენტები
Zachary Bloomgarden, MD
Clinical Professor
Medicine/Endocrinology
Diabetes and Bone Disease
Mount Sinai School of Medicine
New York
NY
გაფრთხილება:
ZB declares that he has no competing interests.
Alicia Jenkins, MB, BS, MD, FRACP, FRCP
Associate Professor
Department of Medicine
University of Melbourne
Melbourne
Australia
Professor
Harold Hamm Oklahoma Diabetes Center
University of Oklahoma Health Sciences Center
Oklahoma City
OK
გაფრთხილება:
AJ has been a (non-salaried) co-investigator on multi-center clinical trials supported by Novo, Eli Lilly, Sanofi-Aventis, and Medtronic. She does not hold any stocks or shares in these companies. She has received a speaker's honorarium from Novo Nordisk.
რეცენზენტების განცხადებები
BMJ Best Practice-ის თემების განახლება სხვადასხვა პერიოდულობით ხდება მტკიცებულებებისა და რეკომენდაციების განვითარების შესაბამისად. ქვემოთ ჩამოთვლილმა რეცენზენტებმა თემის არსებობის მანძილზე კონტენტს ერთხელ მაინც გადახედეს.
გაფრთხილება
რეცენზენტების აფილიაციები და გაფრთხილებები მოცემულია გადახედვის მომენტისთვის.
წყაროები
ძირითადი სტატიები
American Diabetes Association. Standards of care in diabetes - 2025. Diabetes Care. 2025 Jan;48(1):S344-52.სრული ტექსტი
Norris JM, Johnson RK, Stene LC. Type 1 diabetes-early life origins and changing epidemiology. Lancet Diabetes Endocrinol. 2020 Mar;8(3):226-38.სრული ტექსტი აბსტრაქტი
International Society for Pediatric and Adolescent Diabetes. ISPAD clinical practice consensus guidelines 2024. Dec 2024 [nternet publication].სრული ტექსტი
Holt RIG, DeVries JH, Hess-Fischl A, et al. The management of type 1 diabetes in adults. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2021 Dec;64(12):2609-52.სრული ტექსტი აბსტრაქტი
გამოყენებული სტატიები
ამ თემაში მოხსენიებული წყაროების სრული სია ხელმისაწვდომია მომხმარებლებისთვის, რომლებსაც აქვთ წვდომა BMJ Best Practice-ის ყველა ნაწილზე.
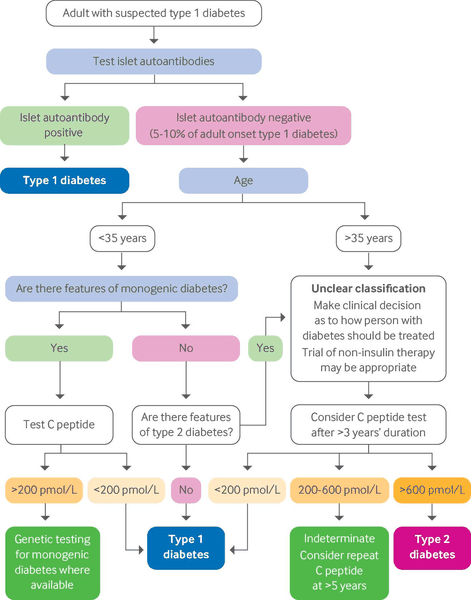
Differentials
- Monogenic diabetes: maturity onset diabetes of the young
- Monogenic diabetes: neonatal diabetes mellitus
- Type 2 diabetes
მეტი DifferentialsGuidelines
- Standards of care in diabetes - 2025
- Management of individuals with diabetes at high risk for hypoglycemia
მეტი GuidelinesPatient information
Diabetes type 1: what is it?
Diabetes type 1: what are the treatment options?
More Patient informationLog in or subscribe to access all of BMJ Best Practice
Use of this content is subject to our disclaimer