Summary
Definition
History and exam
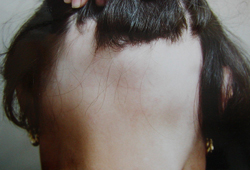
Key diagnostic factors
- hair loss
- nonscarring alopecia
- exclamation point hairs
- normal underlying skin in bare areas
- nail pitting
Other diagnostic factors
- autoimmunity
Risk factors
- autoimmune disease
- family history of autoimmune disease
Diagnostic investigations
1st investigations to order
- clinical diagnosis
- positive hair pull test
Investigations to consider
- skin biopsy
- trichoscopy
Treatment algorithm
limited hair loss (treatment desired)
limited hair loss (no treatment desired)
extensive hair loss (treatment desired)
extensive hair loss (no treatment desired)
Contributors
Authors
Fong Seng Lim, MBBS, MMed (FM), FAMS
Senior Consultant
Department of Family Medicine
National University Health System
Singapore
Disclosures
FSL declares that he has no competing interests.
Miny Samuel, PhD
Assistant Director
Research Support Unit
Yong Loo Lin School of Medicine
National University of Singapore
Singapore
Disclosures
MS declares that she has no competing interests.
Acknowledgements
Dr Fong Seng Lim and Dr Miny Samuel would like to gratefully acknowledge Dr Richard A. Strick, Dr Jack Green, and Dr Paradi Mirmirani, previous contributors to this topic. RAS is an honorary member of the National Alopecia Areata Foundation (NAAF) clinical scientific advisory board. JG is an author of a reference cited in this topic. PM declares that he has no competing interests.
Peer reviewers
Maria Hordinsky, MD
Professor and Chair
Department of Dermatology
University of Minnesota
Minneapolis
MN
Disclosures
MH declares that she has no competing interests.
Ralph M. Trueb, MD
Associate Professor
Department of Dermatology
University Hospital of Zurich
Zurich
Switzerland
Disclosures
RMT is an author of a number of references cited in this topic.
Peer reviewer acknowledgements
BMJ Best Practice topics are updated on a rolling basis in line with developments in evidence and guidance. The peer reviewers listed here have reviewed the content at least once during the history of the topic.
Disclosures
Peer reviewer affiliations and disclosures pertain to the time of the review.
References
Key articles
Olsen EA, Hordinsky MK, Price VH, et al. Alopecia areata investigational assessment guidelines: part II. National Alopecia Areata Foundation. J Am Acad Dermatol. 2004;51:440-447. Abstract
Hordinsky MK. Medical treatment of noncicatricial alopecia. Semin Cutan Med Surg. 2006;25:51-55. Abstract
Messenger AG, McKillop J, Farrant P, et al. British Association of Dermatologists' guidelines for the management of alopecia areata 2012. Br J Dermatol. 2012;166:916-926. Abstract
Reference articles
A full list of sources referenced in this topic is available to users with access to all of BMJ Best Practice.
Differentials
- Trichotillomania
- Tinea capitis
- Telogen effluvium
More DifferentialsGuidelines
- British Association of Dermatologists' guidelines for the management of alopecia areata 2012
- Alopecia areata investigational assessment guidelines: part II
More GuidelinesPatient information
Alopecia areata
More Patient informationLog in or subscribe to access all of BMJ Best Practice
Use of this content is subject to our disclaimer