CAP is diagnosed clinically based on typical symptoms and signs, but these may vary with age and are often fairly nonspecific.[3]Haq IJ, Battersby AC, Eastham K, et al. Community acquired pneumonia in children. BMJ. 2017 Mar 2;356:j686.
http://www.ncbi.nlm.nih.gov/pubmed/28255071?tool=bestpractice.com
[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
A careful history and thorough physical exam are needed to assess severity, identify any risk factors for disease progression, and look for any features that suggest complications (e.g., effusions, empyema).[3]Haq IJ, Battersby AC, Eastham K, et al. Community acquired pneumonia in children. BMJ. 2017 Mar 2;356:j686.
http://www.ncbi.nlm.nih.gov/pubmed/28255071?tool=bestpractice.com
CAP is a common condition in infants and children and is a frequent cause of hospital admission.[3]Haq IJ, Battersby AC, Eastham K, et al. Community acquired pneumonia in children. BMJ. 2017 Mar 2;356:j686.
http://www.ncbi.nlm.nih.gov/pubmed/28255071?tool=bestpractice.com
History
Ask about the baseline health of the child and any underlying comorbidities, the duration and course of symptoms, exposure to sick contacts, immunization status, and recent travel history (as this may have a bearing on potential etiology and patterns of antibiotic resistance). In neonates, check for any maternal health issues or birth complications.
There is no universal presentation of CAP, and no single symptom or sign in isolation is sufficient to indicate CAP.[10]Rees CA, Kuppermann N, Florin TA. Community-acquired pneumonia in children. Pediatr Emerg Care. 2023 Dec 1;39(12):968-76.
http://www.ncbi.nlm.nih.gov/pubmed/38019716?tool=bestpractice.com
Consider the possibility of pneumonia if a child presents with a fever, particularly if associated with one or more of the following: tachypnea; chest crackles; nasal flaring; chest indrawing; cyanosis; oxygen saturation ≤95% on room air.[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
[17]National Institute for Health and Care Excellence. Fever in under 5s: assessment and initial management. Nov 2021 [internet publication].
https://www.nice.org.uk/guidance/ng143
One multicenter study covering 2358 children who had radiographic evidence of pneumonia found that 91% had fever.[4]Jain S, Williams DJ, Arnold SR, et al; CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015 Feb 26;372(9):835-45.
https://www.nejm.org/doi/10.1056/NEJMoa1405870
http://www.ncbi.nlm.nih.gov/pubmed/25714161?tool=bestpractice.com
In addition to fever, other typical symptoms include:[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
Rapid breathing. A raised respiratory rate compared with age-specific norms has been found to correlate well with hypoxemia.[18]Clark JE, Hammal D, Spencer D, et al. Children with pneumonia: how do they present and how are they managed? Arch Dis Child. 2007 May;92(5):394-8.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2083747
http://www.ncbi.nlm.nih.gov/pubmed/17261579?tool=bestpractice.com
Be aware, however, that some children with CAP have a normal respiratory rate.[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
Cough. This is common but not always present, particularly in the early stages of illness.[3]Haq IJ, Battersby AC, Eastham K, et al. Community acquired pneumonia in children. BMJ. 2017 Mar 2;356:j686.
http://www.ncbi.nlm.nih.gov/pubmed/28255071?tool=bestpractice.com
It was present in 95% of children in a multicenter study of 2358 cases of radiographically confirmed CAP.[4]Jain S, Williams DJ, Arnold SR, et al; CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015 Feb 26;372(9):835-45.
https://www.nejm.org/doi/10.1056/NEJMoa1405870
http://www.ncbi.nlm.nih.gov/pubmed/25714161?tool=bestpractice.com
Dyspnea or difficulty breathing. Some 70% of 2358 children with radiographic evidence of pneumonia had dyspnea.[4]Jain S, Williams DJ, Arnold SR, et al; CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015 Feb 26;372(9):835-45.
https://www.nejm.org/doi/10.1056/NEJMoa1405870
http://www.ncbi.nlm.nih.gov/pubmed/25714161?tool=bestpractice.com
Wheeze. Wheeze on its own is a poor indicator of possible CAP and raises suspicion of an alternative diagnosis, such as viral wheeze or an exacerbation of asthma. The presence of wheeze has been found in several studies to be a negative predictor of radiographic CAP.[10]Rees CA, Kuppermann N, Florin TA. Community-acquired pneumonia in children. Pediatr Emerg Care. 2023 Dec 1;39(12):968-76.
http://www.ncbi.nlm.nih.gov/pubmed/38019716?tool=bestpractice.com
[19]Ramgopal S, Ambroggio L, Lorenz D, et al. A prediction model for pediatric radiographic pneumonia. Pediatrics. 2022 Jan 1;149(1):e2021051405.
https://publications.aap.org/pediatrics/article/149/1/e2021051405/183721/A-Prediction-Model-for-Pediatric-Radiographic
http://www.ncbi.nlm.nih.gov/pubmed/34845493?tool=bestpractice.com
[20]Neuman MI, Monuteaux MC, Scully KJ, et al. Prediction of pneumonia in a pediatric emergency department. Pediatrics. 2011 Aug;128(2):246-53.
http://www.ncbi.nlm.nih.gov/pubmed/21746723?tool=bestpractice.com
However, wheeze combined with other typical symptoms can be a pointer toward possible CAP. A study of 526 children evaluated for wheezing in the emergency department found only 4.9% had radiographically confirmed pneumonia.[21]Mathews B, Shah S, Cleveland RH, et al. Clinical predictors of pneumonia among children with wheezing. Pediatrics. 2009 Jul;124(1):e29-36.
http://www.ncbi.nlm.nih.gov/pubmed/19564266?tool=bestpractice.com
However, when wheeze was accompanied by fever 6.9% were found to have radiographic evidence of pneumonia, and when wheeze, fever, and hypoxemia (oxygen saturation <92%) were all present, 20.6% of children had radiographic infiltrates.
Chest pain. This is more commonly reported in older children and adolescents.[5]Shah SN, Bachur RG, Simel DL, et al. Does this child have pneumonia?: the rational clinical examination systematic review. JAMA. 2017 Aug 1;318(5):462-71.
http://www.ncbi.nlm.nih.gov/pubmed/28763554?tool=bestpractice.com
Other symptoms that may be present in some children are:
Abdominal pain. This is occasionally the predominant presenting symptom in children with CAP, especially among those <5 years old.[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
Vomiting.[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
Headache.[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
Difficulty feeding, particularly in infants.
Agitation. This can sometimes be an indicator of hypoxemia.[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
One systematic review of 23 prospective cohort studies involving a total of 13,833 children with suspected pneumonia concluded that no single symptom or sign reliably differentiates CAP from other childhood respiratory illnesses.[5]Shah SN, Bachur RG, Simel DL, et al. Does this child have pneumonia?: the rational clinical examination systematic review. JAMA. 2017 Aug 1;318(5):462-71.
http://www.ncbi.nlm.nih.gov/pubmed/28763554?tool=bestpractice.com
Most but not all children presented with fever, cough, or both. However, hypoxemia and signs of increased work of breathing were found to be most strongly correlated with radiographic evidence of pneumonia.
The authors of the systematic review recommended checking oxygen saturation and carefully observing for evidence of increased work of breathing whenever a child presents with cough and/or fever.
Risk factors for CAP include:
Younger age. Children <2 years old are especially likely to develop CAP and especially complicated CAP (CCAP).[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
[2]de Benedictis FM, Kerem E, Chang AB, et al. Complicated pneumonia in children. Lancet. 2020 Sep 12;396(10253):786-98.
http://www.ncbi.nlm.nih.gov/pubmed/32919518?tool=bestpractice.com
[3]Haq IJ, Battersby AC, Eastham K, et al. Community acquired pneumonia in children. BMJ. 2017 Mar 2;356:j686.
http://www.ncbi.nlm.nih.gov/pubmed/28255071?tool=bestpractice.com
Age <5 years is a risk factor for severe CAP.[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
Male sex. Boys have a higher incidence across all ages.[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
[2]de Benedictis FM, Kerem E, Chang AB, et al. Complicated pneumonia in children. Lancet. 2020 Sep 12;396(10253):786-98.
http://www.ncbi.nlm.nih.gov/pubmed/32919518?tool=bestpractice.com
[3]Haq IJ, Battersby AC, Eastham K, et al. Community acquired pneumonia in children. BMJ. 2017 Mar 2;356:j686.
http://www.ncbi.nlm.nih.gov/pubmed/28255071?tool=bestpractice.com
[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
Prematurity. Prematurity is one of the most important risk factors associated with respiratory diseases. CAP affects preterm infants at a higher rate than full-term infants.[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
[2]de Benedictis FM, Kerem E, Chang AB, et al. Complicated pneumonia in children. Lancet. 2020 Sep 12;396(10253):786-98.
http://www.ncbi.nlm.nih.gov/pubmed/32919518?tool=bestpractice.com
[3]Haq IJ, Battersby AC, Eastham K, et al. Community acquired pneumonia in children. BMJ. 2017 Mar 2;356:j686.
http://www.ncbi.nlm.nih.gov/pubmed/28255071?tool=bestpractice.com
Prematurity is also a risk factor for severe disease.[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
Several chronic conditions. Among the long-term conditions associated with a higher risk of developing CAP, and particularly complicated CAP, are: immunodeficiency; malnutrition; chronic lung disease; congenital heart disease; neurodisability; cerebral palsy; cystic fibrosis; primary ciliary dyskinesia.[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
[2]de Benedictis FM, Kerem E, Chang AB, et al. Complicated pneumonia in children. Lancet. 2020 Sep 12;396(10253):786-98.
http://www.ncbi.nlm.nih.gov/pubmed/32919518?tool=bestpractice.com
[3]Haq IJ, Battersby AC, Eastham K, et al. Community acquired pneumonia in children. BMJ. 2017 Mar 2;356:j686.
http://www.ncbi.nlm.nih.gov/pubmed/28255071?tool=bestpractice.com
[22]O’Connor MG, Mosquera R, Metjian H, et al., Primary ciliary dyskinesia. Chest Pulm. 2023 Jun;1(1):100004.
https://www.chestpulmonary.org/article/S2949-7892(23)00004-1/fulltext
A history of severe and/or complicated and/or recurrent pneumonia. This indicates a higher risk of progression to severe or complicated CAP in a child who presents with mild symptoms.[3]Haq IJ, Battersby AC, Eastham K, et al. Community acquired pneumonia in children. BMJ. 2017 Mar 2;356:j686.
http://www.ncbi.nlm.nih.gov/pubmed/28255071?tool=bestpractice.com
Foreign body inhalation. An undiagnosed and retained inhaled foreign body is a risk factor for CAP and complicated CAP.[2]de Benedictis FM, Kerem E, Chang AB, et al. Complicated pneumonia in children. Lancet. 2020 Sep 12;396(10253):786-98.
http://www.ncbi.nlm.nih.gov/pubmed/32919518?tool=bestpractice.com
Indoor air pollution, caused by cooking and heating with biomass fuels, such as wood or dung.[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
[2]de Benedictis FM, Kerem E, Chang AB, et al. Complicated pneumonia in children. Lancet. 2020 Sep 12;396(10253):786-98.
http://www.ncbi.nlm.nih.gov/pubmed/32919518?tool=bestpractice.com
[3]Haq IJ, Battersby AC, Eastham K, et al. Community acquired pneumonia in children. BMJ. 2017 Mar 2;356:j686.
http://www.ncbi.nlm.nih.gov/pubmed/28255071?tool=bestpractice.com
Living in an overcrowded home. Data suggest that household crowding puts young children at increased risk of acute lower respiratory tract infection because it increases the rate of cross-infection among the family. Pathogens are easily and rapidly transmitted via air droplets and aerosols in crowded and poorly ventilated rooms where people are talking, sneezing, or coughing.[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
[2]de Benedictis FM, Kerem E, Chang AB, et al. Complicated pneumonia in children. Lancet. 2020 Sep 12;396(10253):786-98.
http://www.ncbi.nlm.nih.gov/pubmed/32919518?tool=bestpractice.com
[3]Haq IJ, Battersby AC, Eastham K, et al. Community acquired pneumonia in children. BMJ. 2017 Mar 2;356:j686.
http://www.ncbi.nlm.nih.gov/pubmed/28255071?tool=bestpractice.com
Parental smoking. Children exposed to passive smoking have been found to have an increased likelihood of emergency department attendance and hospital admission for respiratory illness, although these data are not specific for CAP.[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
[2]de Benedictis FM, Kerem E, Chang AB, et al. Complicated pneumonia in children. Lancet. 2020 Sep 12;396(10253):786-98.
http://www.ncbi.nlm.nih.gov/pubmed/32919518?tool=bestpractice.com
[3]Haq IJ, Battersby AC, Eastham K, et al. Community acquired pneumonia in children. BMJ. 2017 Mar 2;356:j686.
http://www.ncbi.nlm.nih.gov/pubmed/28255071?tool=bestpractice.com
An anatomic lesion. A vascular ring or sling (a type of congenital aortic arch anomaly) can result in compression of the trachea and predispose a child to recurrent lower respiratory tract infections.[13]Ctori E, Crucean A, Pinkey B, et al. Morphology of vascular ring arch anomalies influences prognosis and management. Arch Dis Child. 2021 Apr 21;106(5):477-83.
http://www.ncbi.nlm.nih.gov/pubmed/33106229?tool=bestpractice.com
[14]Kellenberger CJ. Aortic arch malformations. Pediatr Radiol. 2010 Jun;40(6):876-84.
http://www.ncbi.nlm.nih.gov/pubmed/20354848?tool=bestpractice.com
There is no reliable way clinically to distinguish between bacterial and viral etiology.[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
Most cases of CAP in infants, toddlers, and preschool children are caused by viruses. Respiratory syncytial virus (RSV) is the most common etiology, detected in 42% of hospitalized patients ages <2 years and 29% of those ages 2-4 years.[4]Jain S, Williams DJ, Arnold SR, et al; CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015 Feb 26;372(9):835-45.
https://www.nejm.org/doi/10.1056/NEJMoa1405870
http://www.ncbi.nlm.nih.gov/pubmed/25714161?tool=bestpractice.com
The other most commonly detected pathogens in hospitalized children in these age groups are human rhinovirus (29% and 25%, respectively) and human metapneumovirus (14% and 17%).[4]Jain S, Williams DJ, Arnold SR, et al; CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015 Feb 26;372(9):835-45.
https://www.nejm.org/doi/10.1056/NEJMoa1405870
http://www.ncbi.nlm.nih.gov/pubmed/25714161?tool=bestpractice.com
In children ages 5-9 years, viral causes still predominate and human rhinovirus is the most frequent pathogen, detected in 30% of hospitalized cases.[4]Jain S, Williams DJ, Arnold SR, et al; CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015 Feb 26;372(9):835-45.
https://www.nejm.org/doi/10.1056/NEJMoa1405870
http://www.ncbi.nlm.nih.gov/pubmed/25714161?tool=bestpractice.com
Among those ages 10-17 years, viral etiologies remain more common than bacterial, with human rhinovirus identified in 19% of hospitalized patients.[4]Jain S, Williams DJ, Arnold SR, et al; CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015 Feb 26;372(9):835-45.
https://www.nejm.org/doi/10.1056/NEJMoa1405870
http://www.ncbi.nlm.nih.gov/pubmed/25714161?tool=bestpractice.com
Bacterial pathogens make up a steadily increasing proportion of cases with increasing age. Streptococcus pneumoniae is the most common typical bacterial pathogen, detected in 4% of all children ages up to 17 years who are hospitalized for CAP.[4]Jain S, Williams DJ, Arnold SR, et al; CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015 Feb 26;372(9):835-45.
https://www.nejm.org/doi/10.1056/NEJMoa1405870
http://www.ncbi.nlm.nih.gov/pubmed/25714161?tool=bestpractice.com
However, atypical infection with Mycoplasma pneumoniae is the most frequent bacterial etiology, detected in 8% of all hospitalized patients (23% of those ages 10-17 years and 16% of those ages 5-9 years, compared with 5% of those ages 2-4 years and 2% of those <2 years).[4]Jain S, Williams DJ, Arnold SR, et al; CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015 Feb 26;372(9):835-45.
https://www.nejm.org/doi/10.1056/NEJMoa1405870
http://www.ncbi.nlm.nih.gov/pubmed/25714161?tool=bestpractice.com
Consider bacterial CAP if the child has a persistent or repetitive fever >101.3°F (>38.5°C) together with chest recession and a raised respiratory rate.[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
The reported incidence of mixed infections ranges from 8.2% to 23%. A prolonged fever in a child with influenza may indicate a secondary bacterial infection.[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
There may be subtle differences in the presentation of CAP associated with specific pathogens.[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
Pneumococcal pneumonia typically starts with fever and tachypnea. Cough is not an initial feature as alveoli have few cough receptors. Cough only begins after lysis occurs and debris irritates airway cough receptors.
Staphylococcal pneumonia is indistinguishable from pneumococcal pneumonia in the early stage of the disease.
Consider the possibility of atypical pneumonia based on local surveillance data.[15]National Institute for Health and Care Excellence. Pneumonia: diagnosis and management. Sep 2025 [internet publication].
https://www.nice.org.uk/guidance/ng250
M pneumoniae infection tends to have peaks or outbreaks every 3-7 years.[23]Diaz MH, Benitez AJ, Winchell JM. Investigations of Mycoplasma pneumoniae infections in the United States: trends in molecular typing and macrolide resistance from 2006 to 2013. J Clin Microbiol. 2015 Jan;53(1):124-30.
https://journals.asm.org/doi/10.1128/jcm.02597-14
http://www.ncbi.nlm.nih.gov/pubmed/25355769?tool=bestpractice.com
Atypical pneumonia caused by M pneumoniae has been reported to account for 8% of CAP hospital admissions in the US.[4]Jain S, Williams DJ, Arnold SR, et al; CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015 Feb 26;372(9):835-45.
https://www.nejm.org/doi/10.1056/NEJMoa1405870
http://www.ncbi.nlm.nih.gov/pubmed/25714161?tool=bestpractice.com
M pneumoniae classically has symptoms that are worse than signs would suggest. Presenting symptoms may be slowly progressing and often include cough that develops over 3-5 days, chest pain, low-grade fever, general malaise, and sometimes arthralgia, sore throat, and headache.[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
[3]Haq IJ, Battersby AC, Eastham K, et al. Community acquired pneumonia in children. BMJ. 2017 Mar 2;356:j686.
http://www.ncbi.nlm.nih.gov/pubmed/28255071?tool=bestpractice.com
[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
[16]Chan SS, Kotecha MK, Rigsby CK, et al; Expert Panel on Pediatric Imaging. ACR appropriateness criteria®: pneumonia in the immunocompetent child. J Am Coll Radiol. 2020 May;17(5 Suppl):S215-25.
https://www.jacr.org/article/S1546-1440(20)30121-6/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/32370966?tool=bestpractice.com
However, a Cochrane review of seven studies covering 1491 children in hospital settings found it is not possible to reliably diagnose pneumonia caused by M pneumoniae based on clinical symptoms and signs.[24]Wang K, Gill P, Perera R, et al. Clinical symptoms and signs for the diagnosis of Mycoplasma pneumoniae in children and adolescents with community-acquired pneumonia. Cochrane Database Syst Rev. 2012 Oct 17;(10):CD009175.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD009175.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/23076954?tool=bestpractice.com
See Atypical pneumonia.
Physical exam
Conduct a thorough physical exam to look for signs that increase confidence in the clinical diagnosis of CAP or are suggestive of severe disease or complications.
Check in particular for hypoxemia (via pulse oximetry) and increased work of breathing (look for grunting; nasal flaring; subcostal, intercostal, or suprasternal chest retractions; and/or head bobbing).[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
These two signs were the most specific indicators of radiographically confirmed CAP in a systematic review of 23 prospective studies involving 13,833 children with suspected pneumonia.[5]Shah SN, Bachur RG, Simel DL, et al. Does this child have pneumonia?: the rational clinical examination systematic review. JAMA. 2017 Aug 1;318(5):462-71.
http://www.ncbi.nlm.nih.gov/pubmed/28763554?tool=bestpractice.com
Oxygen saturation ≤96% on pulse oximetry was found to have a likelihood ratio of 2.8 (95% CI 2.1 to 3.6), a sensitivity of 64%, and a specificity of 77% for pneumonia. Conversely, oxygen saturation >96% was a strong predictor that the child would not have radiographic evidence of pneumonia (likelihood ratio 0.47, 95% CI 0.32 to 0.67).[5]Shah SN, Bachur RG, Simel DL, et al. Does this child have pneumonia?: the rational clinical examination systematic review. JAMA. 2017 Aug 1;318(5):462-71.
http://www.ncbi.nlm.nih.gov/pubmed/28763554?tool=bestpractice.com
Increased work of breathing was found to have a likelihood ratio of 2.1 (95% CI 1.6 to 2.7) for predicting radiographically confirmed pneumonia.[5]Shah SN, Bachur RG, Simel DL, et al. Does this child have pneumonia?: the rational clinical examination systematic review. JAMA. 2017 Aug 1;318(5):462-71.
http://www.ncbi.nlm.nih.gov/pubmed/28763554?tool=bestpractice.com
Apnea may be seen, particularly in infants.[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
Be aware that grunting and cyanosis are signs of severe disease.
Grunting is a sign of impending respiratory failure.[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
Cyanosis is a sign of severe hypoxemia, although it can be difficult to detect.[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
Fever and tachypnea are common but nonspecific signs of CAP.[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
In a systematic review of 23 studies involving 13,833 children with suspected pneumonia:[5]Shah SN, Bachur RG, Simel DL, et al. Does this child have pneumonia?: the rational clinical examination systematic review. JAMA. 2017 Aug 1;318(5):462-71.
http://www.ncbi.nlm.nih.gov/pubmed/28763554?tool=bestpractice.com
Fever >99.5°F (>37.5°C) had a likelihood ratio range of 1.7 to 1.8 for predicting radiographically confirmed CAP (sensitivity 80% to 92%, specificity 47% to 54%).
Tachypnea (respiratory rate [RR] >40 breaths/minute) had a likelihood ratio of 1.5 (95% CI 1.3 to 1.7), sensitivity of 79%, and specificity of 51%.
Tachypnea is a nonspecific sign but correlates well with hypoxemia.[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
[18]Clark JE, Hammal D, Spencer D, et al. Children with pneumonia: how do they present and how are they managed? Arch Dis Child. 2007 May;92(5):394-8.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2083747
http://www.ncbi.nlm.nih.gov/pubmed/17261579?tool=bestpractice.com
One study found that in infants <1 year old, an RR ≥70 breaths/minute had a sensitivity of 63% and specificity of 89% for hypoxemia.[25]Smyth A, Carty H, Hart CA. Clinical predictors of hypoxaemia in children with pneumonia. Ann Trop Paediatr. 1998 Mar;18(1):31-40.
http://www.ncbi.nlm.nih.gov/pubmed/9691999?tool=bestpractice.com
Be aware, however, that some children with CAP have a normal RR.[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
Note that tachypnea is defined according to age-related criteria, although suggested reference ranges for different pediatric age groups vary between different sources. Among children ages ≤5 years, the World Health Organization defines tachypnea as RR (breaths/minute) of: >60 at age 0-2 months; >50 at age 2-12 months; >40 at age 1-5 years.[26]World Health Organization. Recommendations for management of common childhood conditions: evidence for technical update of pocket book recommendations. Geneva, Switzerland: World Health Organization; 2012.
https://iris.who.int/bitstream/handle/10665/44774/9789241502825_eng.pdf
A reasonable cut-off for children ages >5 years is an RR >20 breaths/minute.[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
[27]Fleming S, Thompson M, Stevens R, et al. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: a systematic review of observational studies. Lancet. 2011 Mar 19;377(9770):1011-8.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3789232
http://www.ncbi.nlm.nih.gov/pubmed/21411136?tool=bestpractice.com
[28]McGann KA, Long SS. Respiratory tract symptom complexes. In: Long SS, Prober CG, Fischer M, et al, eds. Principles and practice of pediatric infectious diseases. 6th edition. Philadelphia, PA: Elsevier; 2022:169-77.
Signs of CAP on auscultation may include:[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
Abnormal or decreased breath sounds such as crackles, rales, crepitation, wheeze, and rhonchi. One study found that crackles and bronchial breathing had a sensitivity of 75% and specificity of 57% for pneumonia.[25]Smyth A, Carty H, Hart CA. Clinical predictors of hypoxaemia in children with pneumonia. Ann Trop Paediatr. 1998 Mar;18(1):31-40.
http://www.ncbi.nlm.nih.gov/pubmed/9691999?tool=bestpractice.com
An absence of breath sounds, with a dull percussion note, is suggestive of CAP complicated by pleural effusion.[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
Fremitus is increased in uncomplicated CAP (but reduced if pleural effusion has developed).[2]de Benedictis FM, Kerem E, Chang AB, et al. Complicated pneumonia in children. Lancet. 2020 Sep 12;396(10253):786-98.
http://www.ncbi.nlm.nih.gov/pubmed/32919518?tool=bestpractice.com
Note, however, that a systematic review of 23 studies involving a total of 13,833 children with suspected pneumonia found that no auscultatory finding was significantly associated with a radiographic diagnosis of CAP, perhaps because of the relative subjectivity of auscultatory signs and difficulty interpreting them in children.[5]Shah SN, Bachur RG, Simel DL, et al. Does this child have pneumonia?: the rational clinical examination systematic review. JAMA. 2017 Aug 1;318(5):462-71.
http://www.ncbi.nlm.nih.gov/pubmed/28763554?tool=bestpractice.com
Check the pulse rate for any signs of tachycardia.[3]Haq IJ, Battersby AC, Eastham K, et al. Community acquired pneumonia in children. BMJ. 2017 Mar 2;356:j686.
http://www.ncbi.nlm.nih.gov/pubmed/28255071?tool=bestpractice.com
[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
Check capillary refill time (CRT).
A CRT >2 seconds is considered to be a sign of severe CAP.[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
Assessment of severity and appropriate setting for care
Assess the severity of CAP based on symptoms, signs, and risk factors for severe disease. Also look for any evidence of complications.[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
The assessment of severity will influence decisions on appropriate tests, initial antimicrobial therapy, and route of administration.[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
Look for any signs of sepsis.[2]de Benedictis FM, Kerem E, Chang AB, et al. Complicated pneumonia in children. Lancet. 2020 Sep 12;396(10253):786-98.
http://www.ncbi.nlm.nih.gov/pubmed/32919518?tool=bestpractice.com
[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
See Sepsis in children.
Refer to the hospital for assessment and management if a child has severe pneumonia or pneumonia with suspected complications.[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
Nonsevere pneumonia in previously healthy children can be safely managed in the community.[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
Also take underlying risk factors into account when deciding the appropriate setting for care (e.g., chronic underlying conditions such as congenital heart disease, chronic lung disease of prematurity, cystic fibrosis, bronchiectasis, or immunodeficiency).[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
[17]National Institute for Health and Care Excellence. Fever in under 5s: assessment and initial management. Nov 2021 [internet publication].
https://www.nice.org.uk/guidance/ng143
Be aware that young age is a risk factor for severity of CAP and the need for hospital admission.[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
Arrange hospital admission for any child or infant who has moderate to severe CAP, as indicated by:[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
Sustained peripheral oxygen saturation <90% on room air. This has been shown to be predictive of failure of outpatient oral antibiotic treatment.
Any one or more of the following signs of respiratory distress:
Tachypnea: respiratory rate of >60 breaths/minute at age 0-2 months; >50 at age 2-12 months; >40 at age 1-5 years; >20 at age >5 years.
Dyspnea.
Suprasternal, intercostal, or subcostal retractions, indicating increased work of breathing.
Grunting - a sign of impending respiratory failure.
Nasal flaring or head bobbing.
Apnea.
Cyanosis.
Altered mental status.
Consider hospital admission if:[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
The child is <6 months old
The child is dehydrated, vomiting, or unable to take oral treatments for any other reason
A particularly virulent pathogen such as methicillin-resistant Staphylococcus aureus (MRSA) is suspected or confirmed
There are psychosocial concerns around nonadherence to therapy or difficulty ensuring reliable follow-up.
Look for any symptoms and signs that might suggest complications of CAP (e.g., parapneumonic effusion, empyema, necrotizing pneumonia, lung abscess). Refer to the hospital for assessment and management if these are present.[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
[3]Haq IJ, Battersby AC, Eastham K, et al. Community acquired pneumonia in children. BMJ. 2017 Mar 2;356:j686.
http://www.ncbi.nlm.nih.gov/pubmed/28255071?tool=bestpractice.com
[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
Any child with complicated pneumonia should be treated in a center with specific expertise in this area.[2]de Benedictis FM, Kerem E, Chang AB, et al. Complicated pneumonia in children. Lancet. 2020 Sep 12;396(10253):786-98.
http://www.ncbi.nlm.nih.gov/pubmed/32919518?tool=bestpractice.com
Factors reported to be associated with complicated CAP in previously healthy children include: age <2 years; long prehospital duration of fever; asymmetric chest pain at presentation; iron-deficiency anemia; and pretreatment with ibuprofen and acetaminophen.[2]de Benedictis FM, Kerem E, Chang AB, et al. Complicated pneumonia in children. Lancet. 2020 Sep 12;396(10253):786-98.
http://www.ncbi.nlm.nih.gov/pubmed/32919518?tool=bestpractice.com
However, some of these factors may be confounded by reverse causation.
Suspect effusion if auscultation reveals absent or severely decreased breath sounds with a dull percussion note.[2]de Benedictis FM, Kerem E, Chang AB, et al. Complicated pneumonia in children. Lancet. 2020 Sep 12;396(10253):786-98.
http://www.ncbi.nlm.nih.gov/pubmed/32919518?tool=bestpractice.com
[3]Haq IJ, Battersby AC, Eastham K, et al. Community acquired pneumonia in children. BMJ. 2017 Mar 2;356:j686.
http://www.ncbi.nlm.nih.gov/pubmed/28255071?tool=bestpractice.com
[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
Fremitus is reduced in pleural effusion.[2]de Benedictis FM, Kerem E, Chang AB, et al. Complicated pneumonia in children. Lancet. 2020 Sep 12;396(10253):786-98.
http://www.ncbi.nlm.nih.gov/pubmed/32919518?tool=bestpractice.com
Features that raise suspicion of empyema include:[3]Haq IJ, Battersby AC, Eastham K, et al. Community acquired pneumonia in children. BMJ. 2017 Mar 2;356:j686.
http://www.ncbi.nlm.nih.gov/pubmed/28255071?tool=bestpractice.com
[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
Fever >7 days
Pleuritic chest pain
Severe CAP symptoms
No clinical response to antibiotics after 48 hours
Presence of risk factors (e.g., age >3 years, recent varicella infection).
Children with necrotizing pneumonia usually look ill and have a high fever, cough, and tachypnea that last for several days.[2]de Benedictis FM, Kerem E, Chang AB, et al. Complicated pneumonia in children. Lancet. 2020 Sep 12;396(10253):786-98.
http://www.ncbi.nlm.nih.gov/pubmed/32919518?tool=bestpractice.com
[3]Haq IJ, Battersby AC, Eastham K, et al. Community acquired pneumonia in children. BMJ. 2017 Mar 2;356:j686.
http://www.ncbi.nlm.nih.gov/pubmed/28255071?tool=bestpractice.com
Hypoxia is common.[2]de Benedictis FM, Kerem E, Chang AB, et al. Complicated pneumonia in children. Lancet. 2020 Sep 12;396(10253):786-98.
http://www.ncbi.nlm.nih.gov/pubmed/32919518?tool=bestpractice.com
The child may experience night sweats and produce foul-smelling sputum.[3]Haq IJ, Battersby AC, Eastham K, et al. Community acquired pneumonia in children. BMJ. 2017 Mar 2;356:j686.
http://www.ncbi.nlm.nih.gov/pubmed/28255071?tool=bestpractice.com
Mild anemia and hypoalbuminemia are characteristic.[2]de Benedictis FM, Kerem E, Chang AB, et al. Complicated pneumonia in children. Lancet. 2020 Sep 12;396(10253):786-98.
http://www.ncbi.nlm.nih.gov/pubmed/32919518?tool=bestpractice.com
Pleuritic chest pain may be present.[3]Haq IJ, Battersby AC, Eastham K, et al. Community acquired pneumonia in children. BMJ. 2017 Mar 2;356:j686.
http://www.ncbi.nlm.nih.gov/pubmed/28255071?tool=bestpractice.com
Pleural effusion is often detectable on physical exam.[2]de Benedictis FM, Kerem E, Chang AB, et al. Complicated pneumonia in children. Lancet. 2020 Sep 12;396(10253):786-98.
http://www.ncbi.nlm.nih.gov/pubmed/32919518?tool=bestpractice.com
Staphylococcus aureus, frequently methicillin-resistant strains that produce Panton-Valentine leukocidin (PVL) toxin, is associated with necrotizing pneumonia.[2]de Benedictis FM, Kerem E, Chang AB, et al. Complicated pneumonia in children. Lancet. 2020 Sep 12;396(10253):786-98.
http://www.ncbi.nlm.nih.gov/pubmed/32919518?tool=bestpractice.com
[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
A previous viral respiratory infection can increase the risk for developing necrotizing pneumonia.
Children with lung abscesses usually present with prolonged low-grade fever and cough.[2]de Benedictis FM, Kerem E, Chang AB, et al. Complicated pneumonia in children. Lancet. 2020 Sep 12;396(10253):786-98.
http://www.ncbi.nlm.nih.gov/pubmed/32919518?tool=bestpractice.com
Chest pain, dyspnea, sputum production, and hemoptysis are less common.
Chest exam might be normal or may reveal signs of consolidation.
Initial diagnostic tests: patients managed in the community
Make a clinical diagnosis of CAP without the need for any blood tests, imaging, or microbiologic tests if the symptoms and signs indicate nonsevere disease in an immunocompetent child.[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
[16]Chan SS, Kotecha MK, Rigsby CK, et al; Expert Panel on Pediatric Imaging. ACR appropriateness criteria®: pneumonia in the immunocompetent child. J Am Coll Radiol. 2020 May;17(5 Suppl):S215-25.
https://www.jacr.org/article/S1546-1440(20)30121-6/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/32370966?tool=bestpractice.com
Do not order chest x-rays to confirm suspected CAP in a child who is assessed as well enough to be treated as an outpatient (based on evaluation in the community or in a hospital emergency department). Both US and UK guidelines recommend against chest radiography for children managed as outpatients.[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
Chest radiographs cannot reliably distinguish viral from bacterial CAP and do not have a significant impact on clinical outcomes. They are not needed for outpatients in whom the diagnosis of pneumonia is strongly suspected based on the history and clinical exam.[16]Chan SS, Kotecha MK, Rigsby CK, et al; Expert Panel on Pediatric Imaging. ACR appropriateness criteria®: pneumonia in the immunocompetent child. J Am Coll Radiol. 2020 May;17(5 Suppl):S215-25.
https://www.jacr.org/article/S1546-1440(20)30121-6/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/32370966?tool=bestpractice.com
One Cochrane review found that chest x-rays for children with suspected lower respiratory tract infection led to increased use of antibiotics but without any impact on clinical outcome.[29]Cao AM, Choy JP, Mohanakrishnan LN, et al. Chest radiographs for acute lower respiratory tract infections. Cochrane Database Syst Rev. 2013 Dec 26;(12):CD009119.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD009119.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/24369343?tool=bestpractice.com
Blood cultures and other microbiologic tests are not needed for a fully immunized child with nonsevere CAP.[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
Rapid diagnostic tests for influenza virus and other respiratory viruses may be useful, if available, in the evaluation of children with CAP in outpatient settings.[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
Routine complete blood count (CBC) is not needed in children with suspected CAP who are managed in the community.[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
Initial diagnostic tests: patients managed in the hospital
Avoid routine chest radiography in children referred to the hospital with CAP.[3]Haq IJ, Battersby AC, Eastham K, et al. Community acquired pneumonia in children. BMJ. 2017 Mar 2;356:j686.
http://www.ncbi.nlm.nih.gov/pubmed/28255071?tool=bestpractice.com
[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
There is poor correlation between x-ray appearance and clinical signs and outcome.
The main role of imaging in CAP is to detect complications such as pleural effusion, lung abscess, and bronchopleural fistula.[16]Chan SS, Kotecha MK, Rigsby CK, et al; Expert Panel on Pediatric Imaging. ACR appropriateness criteria®: pneumonia in the immunocompetent child. J Am Coll Radiol. 2020 May;17(5 Suppl):S215-25.
https://www.jacr.org/article/S1546-1440(20)30121-6/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/32370966?tool=bestpractice.com
Reserve chest radiography for any patient who is hospitalized for severe or complicated CAP.[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
[3]Haq IJ, Battersby AC, Eastham K, et al. Community acquired pneumonia in children. BMJ. 2017 Mar 2;356:j686.
http://www.ncbi.nlm.nih.gov/pubmed/28255071?tool=bestpractice.com
[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
It may also be indicated if the child fails to respond to initial outpatient treatment.[16]Chan SS, Kotecha MK, Rigsby CK, et al; Expert Panel on Pediatric Imaging. ACR appropriateness criteria®: pneumonia in the immunocompetent child. J Am Coll Radiol. 2020 May;17(5 Suppl):S215-25.
https://www.jacr.org/article/S1546-1440(20)30121-6/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/32370966?tool=bestpractice.com
Radiographic confirmation of pneumonia is traditionally defined as the presence of a consolidation, opacity, or infiltrate.[5]Shah SN, Bachur RG, Simel DL, et al. Does this child have pneumonia?: the rational clinical examination systematic review. JAMA. 2017 Aug 1;318(5):462-71.
http://www.ncbi.nlm.nih.gov/pubmed/28763554?tool=bestpractice.com
Document the presence, size, and character of parenchymal infiltrates and identify any complications that may require additional interventions over and above antimicrobial therapy and supportive care.[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Chest radiographs confirming pneumonia. Image A: a 6-year-old girl with widespread interstitial changes in both lungs caused by S pneumoniae. Image B: a 1-year-old boy with alveolar changes in the right lower lobe caused by S pneumoniae. Image C: a 2-year-old girl with alveolar changes in the left lower lobe associated with rhinovirus. Image D: a 4-month-old girl with alveolar changes in the right upper lobe associated with parainfluenza 2 and human herpes virusVirkki R, et al. Thorax 2002; 57: 438-41; used with permission [Citation ends].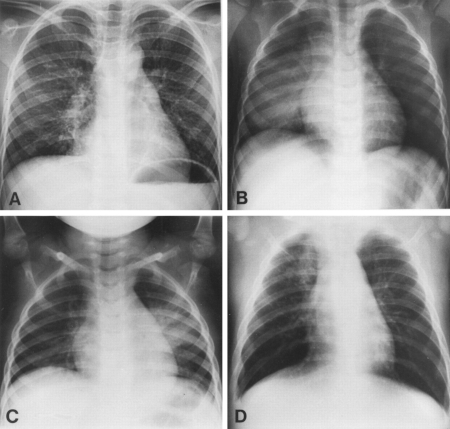
Signs of complications might be revealed by chest x-ray.[2]de Benedictis FM, Kerem E, Chang AB, et al. Complicated pneumonia in children. Lancet. 2020 Sep 12;396(10253):786-98.
http://www.ncbi.nlm.nih.gov/pubmed/32919518?tool=bestpractice.com
Signs of parapneumonic effusion include blunting of the costophrenic angle and a rim of fluid ascending the lateral chest wall (meniscus sign). Large effusions can appear as a complete white-out.
Lung abscess may show as a well defined thick-walled cavity, often containing an air-fluid level. However, in some cases it may be difficult to distinguish an abscess from consolidation.
Note that the initial phase of necrotizing pneumonia is difficult to detect on chest x-ray because fluid-filled cavitary lesions have the same density as adjacent consolidated lung. Chest computed tomography (CT) may be needed.
[Figure caption and citation for the preceding image starts]: Chest x-ray of complicated pneumonia, showing opacification of the left lung field consistent with a large pleural effusion and empyema. There is associated right-sided bronchial wall thickening and consolidationHaq IJ, et al. BMJ 2017 Mar 2; 356: j686. doi: 10.1136/bmj.j686; used with permission [Citation ends].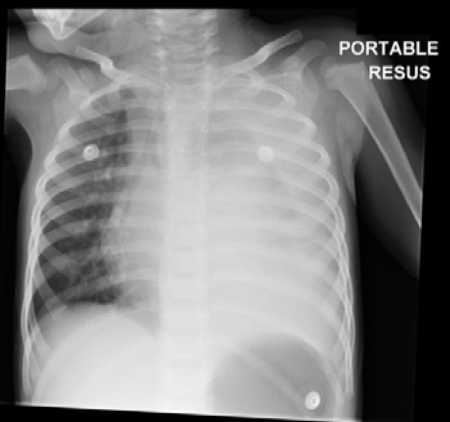
If there is suspicion on chest x-ray of a parapneumonic effusion, chest ultrasound is recommended for confirmation.[16]Chan SS, Kotecha MK, Rigsby CK, et al; Expert Panel on Pediatric Imaging. ACR appropriateness criteria®: pneumonia in the immunocompetent child. J Am Coll Radiol. 2020 May;17(5 Suppl):S215-25.
https://www.jacr.org/article/S1546-1440(20)30121-6/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/32370966?tool=bestpractice.com
Chest ultrasound may also be appropriate for a child who does not respond to initial outpatient treatment.[16]Chan SS, Kotecha MK, Rigsby CK, et al; Expert Panel on Pediatric Imaging. ACR appropriateness criteria®: pneumonia in the immunocompetent child. J Am Coll Radiol. 2020 May;17(5 Suppl):S215-25.
https://www.jacr.org/article/S1546-1440(20)30121-6/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/32370966?tool=bestpractice.com
Ultrasound is more sensitive than chest radiography to evaluate the pleural space.[2]de Benedictis FM, Kerem E, Chang AB, et al. Complicated pneumonia in children. Lancet. 2020 Sep 12;396(10253):786-98.
http://www.ncbi.nlm.nih.gov/pubmed/32919518?tool=bestpractice.com
It can be used to detect small pleural effusions, estimate the size of the effusion, and show any fibrinous septations and can differentiate pleural effusions from consolidated lung.[2]de Benedictis FM, Kerem E, Chang AB, et al. Complicated pneumonia in children. Lancet. 2020 Sep 12;396(10253):786-98.
http://www.ncbi.nlm.nih.gov/pubmed/32919518?tool=bestpractice.com
It can also differentiate empyema from peripheral lung abscess.[2]de Benedictis FM, Kerem E, Chang AB, et al. Complicated pneumonia in children. Lancet. 2020 Sep 12;396(10253):786-98.
http://www.ncbi.nlm.nih.gov/pubmed/32919518?tool=bestpractice.com
Doppler ultrasound can detect necrotic changes before they become apparent on CT.[2]de Benedictis FM, Kerem E, Chang AB, et al. Complicated pneumonia in children. Lancet. 2020 Sep 12;396(10253):786-98.
http://www.ncbi.nlm.nih.gov/pubmed/32919518?tool=bestpractice.com
A wider potential role for bedside lung ultrasound in diagnosis of uncomplicated CAP is under ongoing investigation.[10]Rees CA, Kuppermann N, Florin TA. Community-acquired pneumonia in children. Pediatr Emerg Care. 2023 Dec 1;39(12):968-76.
http://www.ncbi.nlm.nih.gov/pubmed/38019716?tool=bestpractice.com
A meta-analysis of five studies found a sensitivity of 96% and specificity of 93% for diagnosing radiographically confirmed CAP when ultrasound was undertaken by skilled sonographers.[30]Pereda MA, Chavez MA, Hooper-Miele CC, et al. Lung ultrasound for the diagnosis of pneumonia in children: a meta-analysis. Pediatrics. 2015 Apr;135(4):714-22.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9923609
http://www.ncbi.nlm.nih.gov/pubmed/25780071?tool=bestpractice.com
The accuracy of point-of-care ultrasound in the hands of less skilled clinicians remains unclear.[31]Tsou PY, Chen KP, Wang YH, et al. Diagnostic accuracy of lung ultrasound performed by novice versus advanced sonographers for pneumonia in children: a systematic review and meta-analysis. Acad Emerg Med. 2019 Sep;26(9):1074-88.
https://onlinelibrary.wiley.com/doi/10.1111/acem.13818
http://www.ncbi.nlm.nih.gov/pubmed/31211896?tool=bestpractice.com
Further research is needed to determine whether bedside ultrasonography has potential utility for diagnosis of uncomplicated CAP in the emergency department.[10]Rees CA, Kuppermann N, Florin TA. Community-acquired pneumonia in children. Pediatr Emerg Care. 2023 Dec 1;39(12):968-76.
http://www.ncbi.nlm.nih.gov/pubmed/38019716?tool=bestpractice.com
CT chest with intravenous contrast may be useful in limited circumstances in a small subgroup of children with complicated pneumonia, particularly if necrotizing pneumonia is suspected.[2]de Benedictis FM, Kerem E, Chang AB, et al. Complicated pneumonia in children. Lancet. 2020 Sep 12;396(10253):786-98.
http://www.ncbi.nlm.nih.gov/pubmed/32919518?tool=bestpractice.com
[16]Chan SS, Kotecha MK, Rigsby CK, et al; Expert Panel on Pediatric Imaging. ACR appropriateness criteria®: pneumonia in the immunocompetent child. J Am Coll Radiol. 2020 May;17(5 Suppl):S215-25.
https://www.jacr.org/article/S1546-1440(20)30121-6/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/32370966?tool=bestpractice.com
In most children with complicated CAP, chest CT does not provide any useful clinical information to guide management or indicate prognosis over and above that gained from ultrasound.[2]de Benedictis FM, Kerem E, Chang AB, et al. Complicated pneumonia in children. Lancet. 2020 Sep 12;396(10253):786-98.
http://www.ncbi.nlm.nih.gov/pubmed/32919518?tool=bestpractice.com
Reserve chest CT with intravenous contrast for diagnostic doubt (e.g., suspicion of malignancy) or for when appropriate treatment does not lead to clinical improvement.[2]de Benedictis FM, Kerem E, Chang AB, et al. Complicated pneumonia in children. Lancet. 2020 Sep 12;396(10253):786-98.
http://www.ncbi.nlm.nih.gov/pubmed/32919518?tool=bestpractice.com
On chest CT, necrotizing pneumonia will show as a rapid transition from a thin-walled fluid-filled compartment to cavitation. Lung abscess will show as a thick-walled compartment with the fluid, with or without air.[2]de Benedictis FM, Kerem E, Chang AB, et al. Complicated pneumonia in children. Lancet. 2020 Sep 12;396(10253):786-98.
http://www.ncbi.nlm.nih.gov/pubmed/32919518?tool=bestpractice.com
Other diagnostic tests: microbiology
Microbiologic tests are not needed for nonsevere disease.[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
Only seek a microbiologic diagnosis in children:[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
[2]de Benedictis FM, Kerem E, Chang AB, et al. Complicated pneumonia in children. Lancet. 2020 Sep 12;396(10253):786-98.
http://www.ncbi.nlm.nih.gov/pubmed/32919518?tool=bestpractice.com
[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
[15]National Institute for Health and Care Excellence. Pneumonia: diagnosis and management. Sep 2025 [internet publication].
https://www.nice.org.uk/guidance/ng250
With severe disease who are admitted to the hospital
Who have potential complications
Who have suspicion for an unusual pathogen that might require a non-standard antimicrobial regimen
Who fail to respond to initial therapy.
Defining causative pathogens can be challenging.[2]de Benedictis FM, Kerem E, Chang AB, et al. Complicated pneumonia in children. Lancet. 2020 Sep 12;396(10253):786-98.
http://www.ncbi.nlm.nih.gov/pubmed/32919518?tool=bestpractice.com
[3]Haq IJ, Battersby AC, Eastham K, et al. Community acquired pneumonia in children. BMJ. 2017 Mar 2;356:j686.
http://www.ncbi.nlm.nih.gov/pubmed/28255071?tool=bestpractice.com
Clinical and radiologic features do not reliably distinguish bacterial from viral etiology. Moreover, coinfection is common.[7]Chee E, Huang K, Haggie S, et al. Systematic review of clinical practice guidelines on the management of community acquired pneumonia in children. Paediatr Respir Rev. 2022 Jun;42:59-68.
http://www.ncbi.nlm.nih.gov/pubmed/35210170?tool=bestpractice.com
[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
Blood cultures are rarely performed in patients who are managed in the community and demonstrate a poor yield in hospital patients.
Obtaining lower respiratory tract cultures from young children is difficult.
Invasive investigations such as pleural aspiration are reserved for severe cases.
Use of sputum, nasopharyngeal, and oropharyngeal samples is limited by the difficulty in distinguishing colonizing organisms from pathogenic ones. However, these samples can be useful to detect organisms that are almost invariably pathogenic, such as RSV and influenza virus.[2]de Benedictis FM, Kerem E, Chang AB, et al. Complicated pneumonia in children. Lancet. 2020 Sep 12;396(10253):786-98.
http://www.ncbi.nlm.nih.gov/pubmed/32919518?tool=bestpractice.com
Where microbiologic tests are indicated because of severe or complicated disease, consider:
Blood cultures. Only order blood cultures for children who are hospitalized for severe CAP or complicated CAP (ideally before antibiotics are given, if feasible).[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
[2]de Benedictis FM, Kerem E, Chang AB, et al. Complicated pneumonia in children. Lancet. 2020 Sep 12;396(10253):786-98.
http://www.ncbi.nlm.nih.gov/pubmed/32919518?tool=bestpractice.com
[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
Cultures are often negative and in some cases this is because of antibiotic therapy initiated prior to cultures being obtained.[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
Samples are rarely positive; hence, the impact on clinical management is usually small. Nonetheless, culture-directed antimicrobial therapy may be associated with improved clinical outcome in those who do have a pathogen identified.[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
Studies have reported that only around 2.5% to 7% of blood cultures are positive in children hospitalized for CAP.[32]Myers AL, Hall M, Williams DJ, et al. Prevalence of bacteremia in hospitalized pediatric patients with community-acquired pneumonia. Pediatr Infect Dis J. 2013 Jul;32(7):736-40.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3907948
http://www.ncbi.nlm.nih.gov/pubmed/23518826?tool=bestpractice.com
[33]Neuman MI, Hall M, Lipsett SC, et al; Pediatric Research in Inpatient Settings Network. Utility of blood culture among children hospitalized with community-acquired pneumonia. Pediatrics. 2017 Sep;140(3):e20171013.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5574722
http://www.ncbi.nlm.nih.gov/pubmed/28835382?tool=bestpractice.com
This may sometimes be because of prior antibiotic therapy in the community.[2]de Benedictis FM, Kerem E, Chang AB, et al. Complicated pneumonia in children. Lancet. 2020 Sep 12;396(10253):786-98.
http://www.ncbi.nlm.nih.gov/pubmed/32919518?tool=bestpractice.com
Pneumococcal pneumonia is seldom a bacteremic illness, and S pneumoniae is cultured in the blood in <5% of cases.[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
Where this is the case, children who show clear clinical improvement do not need repeat blood cultures to confirm resolution of pneumococcal bacteremia.[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
If blood cultures show bacteremia caused by Staphylococcus aureus, repeat cultures are required to document resolution, regardless of clinical status.[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
Nasopharyngeal swabs for polymerase chain reaction (PCR) multiplex testing.
Rapid diagnostic tests using PCR-based assays can be performed on samples from the nasopharynx, throat, or pleural fluid.[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
This can be particularly useful to avoid inappropriate antibiotic therapy if a viral or atypical bacterial etiology is suspected.[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
Nasopharyngeal secretions are relatively easy to obtain, and the use of PCR testing has been reported to result in pathogen identification in 65% to 83% of cases.[3]Haq IJ, Battersby AC, Eastham K, et al. Community acquired pneumonia in children. BMJ. 2017 Mar 2;356:j686.
http://www.ncbi.nlm.nih.gov/pubmed/28255071?tool=bestpractice.com
Use of rapid multiplex point-of-care PCR tests for individuals who present with respiratory tract infection has become routine in many hospitals since the COVID-19 pandemic. These enable nasal or nasopharyngeal specimens to be tested simultaneously for multiple pathogens, with results available within 1-2 hours.[34]Clark TW, Lindsley K, Wigmosta TB, et al. Rapid multiplex PCR for respiratory viruses reduces time to result and improves clinical care: results of a systematic review and meta-analysis. J Infect. 2023 May;86(5):462-75.
https://www.journalofinfection.com/article/S0163-4453(23)00134-2/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/36906153?tool=bestpractice.com
[35]Ouafi M, Dubos F, Engelmann I, et al. Rapid syndromic testing for respiratory viral infections in children attending the emergency department during COVID-19 pandemic in Lille, France, 2021-2022. J Clin Virol. 2022 Aug;153:105221.
https://www.sciencedirect.com/science/article/pii/S1386653222001548
http://www.ncbi.nlm.nih.gov/pubmed/35777223?tool=bestpractice.com
However, one randomized trial involving 1243 children presenting to an emergency department with fever and/or respiratory symptoms or signs failed to show any significant impact on the proportion of children who were prescribed antibiotics.[36]Mattila S, Paalanne N, Honkila M, et al. Effect of point-of-care testing for respiratory pathogens on antibiotic use in children: a randomized clinical trial. JAMA Netw Open. 2022 Jun 1;5(6):e2216162.
https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2793177
http://www.ncbi.nlm.nih.gov/pubmed/35679047?tool=bestpractice.com
Note that nasopharyngeal culture is uninformative because of the difficulty in distinguishing pathogenic bacteria from normal flora.[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
If an atypical pathogen is suspected, bear in mind the mixed evidence on the value of PCR testing. A study from the Netherlands suggested that nasal PCR testing for M pneumoniae has similar rates of positivity in symptomatic children versus asymptomatic carriers, although a subsequent US study failed to support this observation.[4]Jain S, Williams DJ, Arnold SR, et al; CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015 Feb 26;372(9):835-45.
https://www.nejm.org/doi/10.1056/NEJMoa1405870
http://www.ncbi.nlm.nih.gov/pubmed/25714161?tool=bestpractice.com
[37]Spuesens EB, Fraaij PL, Visser EG, et al. Carriage of Mycoplasma pneumoniae in the upper respiratory tract of symptomatic and asymptomatic children: an observational study. PLoS Med. 2013;10(5):e1001444.
https://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1001444
http://www.ncbi.nlm.nih.gov/pubmed/23690754?tool=bestpractice.com
See Atypical pneumonia.
Be aware that identification of a viral etiology (e.g., influenza) does not exclude a bacterial pathogen because of the high incidence of coinfection.
Consider obtaining a sputum sample for culture and Gram stain if an older child or adolescent has been hospitalized with severe CAP.[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
If pleural fluid is obtained (e.g., because of severe CAP or evidence of pleural effusion), send it for:[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
[2]de Benedictis FM, Kerem E, Chang AB, et al. Complicated pneumonia in children. Lancet. 2020 Sep 12;396(10253):786-98.
http://www.ncbi.nlm.nih.gov/pubmed/32919518?tool=bestpractice.com
[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
Microscopy and culture (including Gram staining, acid-fast bacilli staining, Mycobacterium tuberculosis testing, antibiotic sensitivity testing).
Pneumococcal antigen detection and/or PCR. PCR on pleural fluid is more specific and more sensitive than PCR on blood samples, and pleural fluid testing for pneumococcal antigen has a high positive predictive value for pneumococcal empyema.[2]de Benedictis FM, Kerem E, Chang AB, et al. Complicated pneumonia in children. Lancet. 2020 Sep 12;396(10253):786-98.
http://www.ncbi.nlm.nih.gov/pubmed/32919518?tool=bestpractice.com
If the child has a lymphocytic effusion or risk factors for tuberculosis (TB), or lives in an area with high incidence of TB, test for M tuberculosis, using induced sputum if feasible or PCR testing of a nasopharyngeal sample.[2]de Benedictis FM, Kerem E, Chang AB, et al. Complicated pneumonia in children. Lancet. 2020 Sep 12;396(10253):786-98.
http://www.ncbi.nlm.nih.gov/pubmed/32919518?tool=bestpractice.com
If a child requires mechanical ventilation for severe or life-threatening CAP, obtain tracheal aspirates at the time of endotracheal tube placement. Send them for Gram stain and culture and for guided testing for viral pathogens.[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
Reserve bronchoscopy with bronchoalveolar lavage (BAL) for immunocompetent children with severe CAP whose initial diagnostic tests fail to yield any positive results.[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
BAL is complex in children, particularly neonates, because of small airways.
Flexible bronchoscopy is well tolerated. However, this test is only available in some centers and has a small pathogenic yield in children who are not immunosuppressed.[2]de Benedictis FM, Kerem E, Chang AB, et al. Complicated pneumonia in children. Lancet. 2020 Sep 12;396(10253):786-98.
http://www.ncbi.nlm.nih.gov/pubmed/32919518?tool=bestpractice.com
Do not use urinary antigen detection tests for diagnosis of pneumococcal pneumonia.[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
Other diagnostic tests: laboratory
Order a CBC, serum electrolytes, blood urea nitrogen (BUN), and liver function tests for any patient with severe pneumonia. Interpret the results in the context of the clinical exam and other laboratory and imaging studies.[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
[9]Harris M, Clark J, Coote N, et al; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011 Oct;66 Suppl 2:ii1-23.
https://www.brit-thoracic.org.uk/quality-improvement/clinical-resources/paediatric-community-acquired-pneumonia
http://www.ncbi.nlm.nih.gov/pubmed/21903691?tool=bestpractice.com
WBC count is typically elevated, but the degree of elevation does not reliably distinguish bacterial from viral infection.
The presence of anemia or thrombocytopenia may raise concern for hemolytic-uremic syndrome, a rare complication of pneumococcal pneumonia.
Measurement of serum electrolytes may be helpful in assessing hydration status in children with reduced fluid intake. Hyponatremia is common in children with CAP.[2]de Benedictis FM, Kerem E, Chang AB, et al. Complicated pneumonia in children. Lancet. 2020 Sep 12;396(10253):786-98.
http://www.ncbi.nlm.nih.gov/pubmed/32919518?tool=bestpractice.com
Acute phase reactants (WBC count, procalcitonin, C-reactive protein [CRP], erythrocyte sedimentation rate [ESR]) are unreliable for distinguishing bacterial from viral etiology. However, they can be useful in severe CAP when measured serially to monitor response to treatment.[2]de Benedictis FM, Kerem E, Chang AB, et al. Complicated pneumonia in children. Lancet. 2020 Sep 12;396(10253):786-98.
http://www.ncbi.nlm.nih.gov/pubmed/32919518?tool=bestpractice.com
Results must be interpreted in the context of the clinical exam and other laboratory and imaging studies.[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
Recommendations on measuring acute phase reactants vary, so check your local protocol.
In the US, the PIDS/IDSA 2011 guideline:[1]Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011 Oct;53(7):e25-76.
https://academic.oup.com/cid/article/53/7/e25/424286
http://www.ncbi.nlm.nih.gov/pubmed/21880587?tool=bestpractice.com
Recommends against using ESR, CRP, and serum procalcitonin as the sole means to distinguish viral from bacterial causes of CAP.
States that measurement of ESR, CRP, and procalcitonin is not necessary for fully immunized children with suspected CAP managed in the community but may provide useful information for those with more severe disease.
States that elevated procalcitonin can be a marker of serious bacterial infection, although values vary widely. If procalcitonin is low, this can be a pointer toward viral pneumonia. If a child with a confirmed viral pneumonia has elevated procalcitonin, this can raise suspicion of bacterial coinfection.
Recommends to consider using serial results in conjunction with clinical findings to assess response to therapy, because declining levels of CRP or procalcitonin may correlate with improved clinical symptoms.
Subsequent findings have suggested that a low procalcitonin level has a high negative predictive value for bacterial CAP.[38]Stockmann C, Ampofo K, Killpack J, et al. Procalcitonin accurately identifies hospitalized children with low risk of bacterial community-acquired pneumonia. J Pediatric Infect Dis Soc. 2018 Feb 19;7(1):46-53.
https://academic.oup.com/jpids/article/7/1/46/2966998
http://www.ncbi.nlm.nih.gov/pubmed/28158460?tool=bestpractice.com
[39]Florin TA, Williams DJ. PRO: Procalcitonin has clinical utility in children with community-acquired pneumonia. JAC Antimicrob Resist. 2021 Dec;3(4):dlab158.
https://academic.oup.com/jacamr/article/3/4/dlab158/6407655
http://www.ncbi.nlm.nih.gov/pubmed/36275888?tool=bestpractice.com
Procalcitonin levels <0.25 nanograms/mL can accurately identify children at lower risk of bacterial CAP, for whom antibiotics are unlikely to be helpful.[7]Chee E, Huang K, Haggie S, et al. Systematic review of clinical practice guidelines on the management of community acquired pneumonia in children. Paediatr Respir Rev. 2022 Jun;42:59-68.
http://www.ncbi.nlm.nih.gov/pubmed/35210170?tool=bestpractice.com
[38]Stockmann C, Ampofo K, Killpack J, et al. Procalcitonin accurately identifies hospitalized children with low risk of bacterial community-acquired pneumonia. J Pediatric Infect Dis Soc. 2018 Feb 19;7(1):46-53.
https://academic.oup.com/jpids/article/7/1/46/2966998
http://www.ncbi.nlm.nih.gov/pubmed/28158460?tool=bestpractice.com
[40]Tsou PY, Rafael J, Ma YK, et al. Diagnostic accuracy of procalcitonin for bacterial pneumonia in children - a systematic review and meta-analysis. Infect Dis (Lond). 2020 Oct;52(10):683-97.
http://www.ncbi.nlm.nih.gov/pubmed/32615062?tool=bestpractice.com




