Summary
Definition
History and exam
Key diagnostic factors
- history of traumatic or nontraumatic cutaneous lesion
- anesthesia or severe pain over site of cellulitis
- fever
- palpitations, tachycardia, tachypnea, hypotension, and lightheadedness
- nausea and vomiting
- delirium
- crepitus
- vesicles or bullae
- gray discoloration of skin
- edema or induration
- location of lesion
Risk factors
- inpatient contact with index case
- Varicella zoster infection
- cutaneous injury, surgery, trauma
- nontraumatic skin lesions
- intravenous drug use
- chronic illness
- immunosuppression
- use of nonsteroidal anti-inflammatory drugs (NSAIDs)
Diagnostic investigations
1st investigations to order
- surgical exploration
- blood and tissue cultures
- gram stain
- complete blood count and differential
- serum electrolytes
- serum BUN and creatinine
- serum CRP
- serum creatine kinase (CK)
- serum lactate
- clotting screen
- arterial blood gas
Investigations to consider
- radiography, CT/MRI, ultrasound
- fresh frozen section
Treatment algorithm
suspected necrotizing fasciitis, organism unknown
type I necrotizing fasciitis (polymicrobial)
type II necrotizing fasciitis due to group A streptococcus
type II necrotizing fasciitis due to Staphylococcus aureus
type II necrotizing fasciitis due to Vibrio vulnificus
type II necrotizing fasciitis due to Aeromonas hydrophila
type II necrotizing fasciitis due to mucorales
persistent cosmetic and functional defects after debridement
Contributors
Expert advisers
Ramia Zakhour, MD
Assistant Professor
Department of Pediatrics
University of Texas
McGovern Medical School
Houston
TX
利益声明
RZ declares that they have no competing interests.
鸣谢
Dr Ramia Zakour would like to gratefully acknowledge Dr Kevin Steiner and Dr William Petri, previous contributors to this topic.
Declarações
KS and WP declared they have no competing interests.
Revisores
Felix Lui, MD, FACS
Associate Professor of Surgery
Yale School of Medicine
New Haven
VT
Declarações
FL declares that he has no competing interests.
Shiranee Sriskandan, MA, MBBChir, FRCP, PhD
Professor of Infectious Diseases and Hon. Consultant
Section of Infectious Diseases
Imperial College London
London
UK
Declarações
SS declares that she has no competing interests.
Créditos aos pareceristas
Os tópicos do BMJ Best Practice são constantemente atualizados, seguindo os desenvolvimentos das evidências e das diretrizes. Os pareceristas aqui listados revisaram o conteúdo pelo menos uma vez durante a história do tópico.
Declarações
As afiliações e declarações dos pareceristas referem--se ao momento da revisão.
Referências
Principais artigos
Sartelli M, Guirao X, Hardcastle TC, et al. 2018 WSES/SIS-E consensus conference: recommendations for the management of skin and soft-tissue infections. World J Emerg Surg. 2018 Dec 14;13:58.Texto completo Resumo
Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014 Jul 15;59(2):e10-52.Texto completo Resumo
Artigos de referência
Uma lista completa das fontes referenciadas neste tópico está disponível para os usuários com acesso total ao BMJ Best Practice.
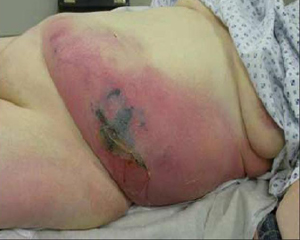
Diagnósticos diferenciais
- Cellulitis
- Impetigo
- Erysipelas
Mais Diagnósticos diferenciaisDiretrizes
- WSES/GAIS/WSIS/SIS-E/AAST global clinical pathways for patients with skin and soft tissue infections
- 2018 WSES/SIS-E consensus conference: recommendations for the management of skin and soft-tissue infections
Mais DiretrizesConectar-se ou assinar para acessar todo o BMJ Best Practice
O uso deste conteúdo está sujeito ao nosso aviso legal