Summary
Definition
History and exam
Key diagnostic factors
- history of drug exposure
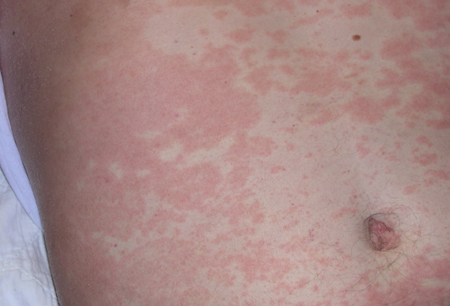
- skin lesions
- variable skin reactions within 5 to 15 minutes of drug exposure
- variable skin reactions within a few hours of drug exposure
- variable skin reactions within 2 weeks of drug exposure
- variable skin reactions within months to years of drug exposure
- previous exposure and reaction to drug
Other diagnostic factors
- pruritus
- associated noncutaneous features
- pain
Risk factors
- virus infections
- HIV infection
- HLA-B*5701 polymorphism
- HLA-B*1502 polymorphism
- HLA-B*5801 polymorphism
- female sex
Diagnostic tests
Tests to avoid
- immunoglobulin G (IgG) allergy testing
Tests to consider
- blood (whole blood, plasma, serum) drug concentration
- serum tryptase concentration (anaphylaxis)
- complement pathway assay
- histology of lesion biopsy
- CBC and differential
- antihistone antibodies to single-stranded DNA (lupus-like syndrome)
- skin tests (prick tests, intradermal tests, patch tests)
- drug-specific IgE
Emerging tests
- basophil activation test
- lymphocyte proliferation assay (LPA/LTT)
- enzyme-linked immunospot assay (ELISPOT test)
Treatment algorithm
serious cutaneous adverse reactions
nonserious cutaneous adverse reactions
following acute episode
Contributors
Authors
Michael Ardern-Jones, BSc, MBBS, DPhil, FRCP
Associate Professor
Consultant Dermatologist
Faculty of Medicine
University of Southampton
Southampton
UK
Disclosures
MA-J declares that he has no competing interests.
Acknowledgements
Dr Michael Ardern-Jones would like to gratefully acknowledge Dr Wei Yann Haw, Dr Anne Holbrook, Dr Hermenio Lima, and Dr Jeffrey K. Aronson, the previous contributors to this topic.
Disclosures
WYH, AH and HL declare that they have no competing interests. JKA is editor of Meyler's Side Effects of Drugs and its annual companion volumes, the Side Effects of Drugs Annuals.
Peer reviewers
Shahbaz A. Janjua, MD
Specialist Dermatologist
Ayza Skin & Research Center
Lalamusa
Pakistan
Disclosures
SAJ declares that he has no competing interests.
Craig K. Svensson, Pharm.D, PhD
Dean
College of Pharmacy, Nursing, and Health Sciences
Purdue University
West Lafayette
IN
Disclosures
CKS declares that he has no competing interests.
Peer reviewer acknowledgements
BMJ Best Practice topics are updated on a rolling basis in line with developments in evidence and guidance. The peer reviewers listed here have reviewed the content at least once during the history of the topic.
Disclosures
Peer reviewer affiliations and disclosures pertain to the time of the review.
References
Key articles
Aronson JK, Ferner RE. Joining the DoTS: new approach to classifying adverse drug reactions. BMJ. 2003 Nov 22;327(7425):1222-5. Abstract
Gell PG, Coombs RRA, eds. Clinical aspects of immunology. 1st ed. Oxford: Blackwell; 1963.
Bigby M, Jick S, Jick H, et al. Drug-induced cutaneous reactions: a report from the Boston Collaborative Drug Surveillance Program on 15,438 consecutive inpatients, 1975 to 1982. JAMA. 1986 Dec 26;256(2):3358-63. Abstract
Kuokkanen K. Drug eruptions: a series of 464 cases in the Department of Dermatology, University of Turku, Finland, during 1966-1970. Acta Allergol. 1972 Dec;27(5):407-38. Abstract
Aronson JK, Ferner RE. Clarification of terminology in drug safety. Drug Saf. 2005;28(10):851-70. Abstract
Khan DA, Banerji A, Blumenthal KG, et al. Drug allergy: a 2022 practice parameter update. J Allergy Clin Immunol. 2022 Dec;150(6):1333-93.Full text Abstract
Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981 Aug;30(2):239-45. Abstract
Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965 Jan;58(1):295-300.Full text Abstract
Howick J, Glasziou P, Aronson JK. The evolution of evidence hierarchies: what can Bradford Hill's 'guidelines for causation' contribute? J R Soc Med. 2009 May;102(5):186-94.Full text Abstract
National Institute for Health and Care Excellence. Drug allergy: diagnosis and management. September 2014 [internet publication].Full text
Reference articles
A full list of sources referenced in this topic is available to users with access to all of BMJ Best Practice.
Differentials
- Systemic lupus erythematosus
- Autoimmune blistering disorders
- Staphylococcal scalded skin syndrome
More DifferentialsGuidelines
- Anaphylaxis: a 2023 practice parameter update
- Drug allergy: a 2022 practice parameter
More GuidelinesLog in or subscribe to access all of BMJ Best Practice
Use of this content is subject to our disclaimer