Резюме
Определение
Анамнез и осмотр
Ключевые диагностические факторы
- calf swelling
- localized pain along deep venous system
Другие диагностические факторы
- asymmetric edema
- prominent superficial veins
- swelling of the entire leg
- phlegmasia cerulea dolens
Факторы риска
- recently bed-bound for 3 days or more
- major surgery within the preceding 3 months
- medical hospitalization within the preceding 2 months
- active cancer
- previous venous thromboembolic event
- recent trauma or fracture
- increasing age
- pregnancy and the postpartum
- varicose veins
- paralysis of the lower extremities
- hereditary thrombophilias
- factor V Leiden
- prothrombin gene G20210A mutation
- protein C or protein S deficiency
- antithrombin deficiency
- antiphospholipid syndrome
- medical comorbidity
- use of specific drugs
- obesity
- cigarette smoking
- recent long-duration air travel
- family history of venous thromboembolism
- central venous catheterization
Диагностические исследования
Исследования, которые показаны в первую очередь
- Wells score
- quantitative D-dimer level
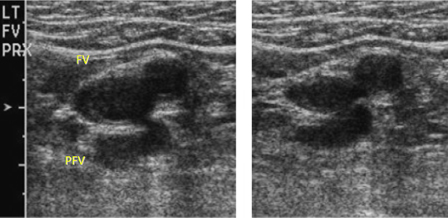
- venous duplex ultrasound (DUS)
- INR and activated partial thromboplastin time (aPTT)
- BUN and creatinine
- LFTs
- CBC
Исследования, проведение которых нужно рассмотреть
- Doppler venous flow testing
- CT abdomen and pelvis with contrast
- thrombophilia screen
Алгоритм лечения
suspected or confirmed DVT of the leg with phlegmasia cerulea dolens
suspected or confirmed DVT without phlegmasia cerulea dolens and no contraindications to anticoagulation: initiation-phase therapy
suspected or confirmed DVT without phlegmasia cerulea dolens: contraindications to anticoagulation
confirmed DVT of the leg: treatment-phase therapy
provoked DVT: extended-phase therapy
unprovoked DVT: extended-phase therapy
pregnant: extended-phase therapy
cancer-associated: extended-phase therapy
recurrent VTE: extended-phase therapy
Составители
Эксперты-консультанты
Scott M. Stevens, MD
Director
Thrombosis Clinic
Intermountain Medical Center
Murray
Professor of Medicine
Department of Medicine
Intermountain Healthcare and University of Utah
Salt Lake City
UT
Раскрытие информации
SMS declares that he has no competing interests.
Scott C. Woller, MD
Director
Thrombosis Clinic
Intermountain Medical Center
Murray
Professor of Medicine
Department of Medicine
Intermountain Healthcare and University of Utah
Salt Lake City
UT
Раскрытие информации
SCW declares that he is expecting to receive funding of an investigator-initiated grant from Janssen Pharmaceuticals to Intermountain Health with no direct compensation to himself for research in the sum of $500,000 in 2024.
Gabriel V. Fontaine, PharmD, MBA, BCPS
Clinical Pharmacy Manager
Critical Care and Emergency Medicine
Advanced Clinical Pharmacist
Neuroscience Critical Care
Intermountain Medical Center
Murray
UT
Раскрытие информации
GVF has received consulting fees and honoraria from AstraZeneca, Chiesi, and Anticoagulation Forum.
Выражение благодарностей
Dr Scott M. Stevens, Dr Scott C. Woller, and Dr Gabriel V. Fontaine would like to gratefully acknowledge Dr Geno Merli, Dr Taki Galanis, Dr Luis Eraso, Dr Geoffrey Ouma, Dr Richard White, and Dr Windsor Ting, the previous contributors to this topic.
Раскрытие информации
GM has received grant or research support from BMS, J&J, Sanofi-Aventis, Portola, and Janssen; he has served as a Scientific Consultant for BMS, J&J, and Sanofi-Aventis. RW declares participation in numerous multicentered clinical trials sponsored by companies: Agenix, Boehringer-Ingelheim, Amgen, Bayer, Bristol-Meyer-Squibb, Novartis, Hemosense. TG, LE, GO, and WT declare that they have no competing interests.
Рецензенты
Beverly Hunt, FRCP, FRCPath, MD
Professor of Thrombosis & Haemostasis
King's College
Consultant
Departments of Haematology, Pathology & Rheumatology
Lead in Blood Sciences
Guy's & St Thomas' NHS Foundation Trust
London
UK
Declarações
BH declares that she has no competing interests.
Créditos aos pareceristas
Os tópicos do BMJ Best Practice são constantemente atualizados, seguindo os desenvolvimentos das evidências e das diretrizes. Os pareceristas aqui listados revisaram o conteúdo pelo menos uma vez durante a história do tópico.
Declarações
As afiliações e declarações dos pareceristas referem--se ao momento da revisão.
Referências
Principais artigos
Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021 Dec;160(6):e545-608.Texto completo Resumo
Mazzolai L, Ageno W, Alatri A, et al. Second consensus document on diagnosis and management of acute deep vein thrombosis: updated document elaborated by the ESC Working Group on aorta and peripheral vascular diseases and the ESC Working Group on pulmonary circulation and right ventricular function. Eur J Prev Cardiol. 2022 May 27;29(8):1248-63.Texto completo Resumo
Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012 Feb;141(2 suppl):e195S-226S.Texto completo Resumo
Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: compendium and review of CHEST guidelines 2012-2021. Chest. 2024 Aug;166(2):388-404.Texto completo Resumo
Artigos de referência
Uma lista completa das fontes referenciadas neste tópico está disponível para os usuários com acesso total ao BMJ Best Practice.
დიფერენციული დიაგნოზები
- Cellulitis
- Calf muscle tear/Achilles tendon tear
- Calf muscle hematoma
მეტი დიფერენციული დიაგნოზებიგაიდლაინები
- NCCN clinical practice guidelines in oncology: cancer-associated venous thromboembolic disease
- ACR-AIUM-SPR-SRU practice parameter for the performance of peripheral venous ultrasound examination
მეტი გაიდლაინებიკალკულატორები
Pretest Probability of Heparin Induced Thrombocytopenia (4-T's score)
მეტი კალკულატორებიპაციენტის ბროშურები
Deep vein thrombosis
DVT and long-distance travel
მეტი პაციენტის ბროშურებიშედით სისტემაში ან გამოიწერეთ BMJ Best Practice
ამ მასალის გამოყენება ექვემდებარება ჩვენს განცხადებას