小结
定义
病史和体格检查
关键诊断因素
- first trimester of pregnancy
- vaginal bleeding
- unusual uterine size for gestational age
其他诊断因素
- early-onset preeclampsia
- shortness of breath and respiratory distress
- severe nausea and emesis
- tachycardia, tremor, insomnia, and diarrhea
- pallor
- pelvic pain
- uterine bleeding
危险因素
- extremes of maternal age
- prior molar pregnancy
- diminished dietary fat and carotene
诊断性检查
首要检查
- histologic exam of placental tissue
- serum human chorionic gonadotropin (hCG)
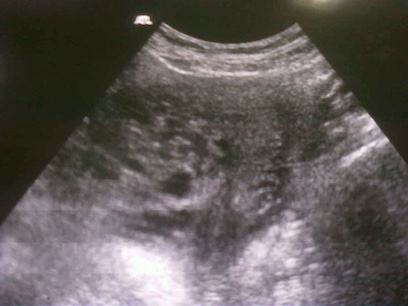
- pelvic ultrasound
需考虑的检查
- CBC
- serum PT, PTT
- serum metabolic panel
- serum thyroid-stimulating hormone (TSH)
- blood type with antibody screen
- CXR
治疗流程
singleton molar pregnancy: desiring fertility
singleton molar pregnancy: not desiring fertility
viable twin fetus: elective termination not desired
viable twin fetus: elective termination
following initial management: high risk of gestational trophoblastic neoplasia with completed follow up unlikely
撰稿人
Authors
Antonio Braga, MD
Associate Professor
Department of Obstetrics and Gynecology
Rio de Janeiro Federal University
Department of Maternal Child
Fluminense Federal University
Rio de Janeiro
Brazil
Disclosures
AB is an author of references cited in this topic.
Kevin M. Elias, MD
Assistant Professor
Brigham and Women’s Hospital
Dana Farber Cancer Institute
New England Trophoblastic Disease Center
Division of Gynecologic Oncology
Department of Obstetrics, Gynecology and Reproductive Biology
Harvard Medical School
Boston
MA
Disclosures
KME has been a paid consultant for AOA Dx and received research support from Aspira Women’s Health and Abcam, Inc. KME is an author of references cited in this topic.
Neil S. Horowitz, MD
Associate Professor
Brigham and Women’s Hospital
Dana Farber Cancer Institute
New England Trophoblastic Disease Center
Division of Gynecologic Oncology
Department of Obstetrics, Gynecology and Reproductive Biology
Harvard Medical School
Boston
MA
Disclosures
NSH is an author of references cited in this topic.
Ross S. Berkowitz, MD
William H. Baker Professor of Gynecology
Brigham and Women’s Hospital
Dana Farber Cancer Institute
New England Trophoblastic Disease Center
Division of Gynecologic Oncology
Department of Obstetrics, Gynecology and Reproductive Biology
Harvard Medical School
Boston
MA
Disclosures
RSB is an author of references cited in this topic.
Acknowledgements
Dr Ross S. Berkowitz, Dr Kevin M. Elias, Dr Neil S. Horowitz, and Dr Antonio Braga would like to gratefully acknowledge Dr John Soper and Dr Emma Rossi, previous contributors to this topic. JS and ER declare that they have no competing interests.
Peer reviewers
Jane Stewart, PhD, MSc
Consultant Gynecologist
Subspecialist in Reproductive Medicine
Newcastle Fertility Centre at Life
Bioscience Centre
International Centre at Life
Newcastle upon Tyne
UK
Disclosures
JS declares that she has no competing interests.
Philip Savage, PhD, FRCP
Consultant in Medical Oncology
Department of Medical Oncology
Charing Cross Hospital
London
UK
Disclosures
PS declares that he has no competing interests.
Aparna Sundaram, DO, MBA, MPH
Physician Consultant
Preventive Medicine
Private Practice
Atlanta
GA
Disclosures
AS declares that she has no competing interests.
Peer reviewer acknowledgements
BMJ Best Practice topics are updated on a rolling basis in line with developments in evidence and guidance. The peer reviewers listed here have reviewed the content at least once during the history of the topic.
Disclosures
Peer reviewer affiliations and disclosures pertain to the time of the review.
References
Key articles
Horowitz NS, Eskander RN, Adelman MR, et al. Epidemiology, diagnosis, and treatment of gestational trophoblastic disease: A Society of Gynecologic Oncology evidenced-based review and recommendation. Gynecol Oncol. 2021 Dec;163(3):605-13.Full text Abstract
Ngan HYS, Seckl MJ, Berkowitz RS, et al. Diagnosis and management of gestational trophoblastic disease: 2021 update. Int J Gynaecol Obstet. 2021 Oct;155 Suppl 1(suppl 1):86-93.Full text Abstract
Tidy, J, Seckl, M, Hancock, BW, on behalf of the Royal College of Obstetricians and Gynaecologists. Management of gestational trophoblastic disease. BJOG 2021;128: e1-e27.Full text
Lok C, van Trommel N, Massuger L, et al. Practical clinical guidelines of the EOTTD for treatment and referral of gestational trophoblastic disease. Eur J Cancer. 2020 May;130:228-40. Abstract
Reference articles
A full list of sources referenced in this topic is available to users with access to all of BMJ Best Practice.
Differentials
- Hyperemesis gravidarum
- Spontaneous abortion
- Multiple gestation
More DifferentialsGuidelines
- Epidemiology, diagnosis, and treatment of gestational trophoblastic disease
- Diagnosis and treatment of gestational trophoblastic disease
More GuidelinesLog in or subscribe to access all of BMJ Best Practice
Use of this content is subject to our disclaimer