Resumen
Definición
Anamnesis y examen
Principales factores de diagnóstico
- presence of risk factors
- abnormal vaginal bleeding
- postcoital bleeding
- pelvic or back pain
- dyspareunia
- cervical mass
- cervical bleeding
Otros factores de diagnóstico
- mucoid or purulent vaginal discharge
- bladder, renal, or bowel obstruction
- bone pain
Factores de riesgo
- human papillomavirus (HPV) infection
- age group
- HIV infection
- early onset of sexual activity (younger than 18)
- multiple sexual partners
- cigarette smoking
- immunosuppression
- in utero exposure to diethylstilbestrol
- history of STI
- oral contraceptive pill use
- high parity
- uncircumcised male partner
- micronutrient malnutrition
- low serum folate
- low vitamin C and E levels
- alcohol use
- low socioeconomic status
Pruebas diagnósticas
Primeras pruebas diagnósticas para solicitar
- vaginal or speculum examination
- colposcopy
- biopsy
- human papillomavirus (HPV) testing
Pruebas diagnósticas que deben considerarse
- FBC
- renal function testing
- liver function tests
- chest x-ray
- intravenous pyelogram
- renal ultrasound
- barium enema
- sigmoidoscopy
- cystoscopy
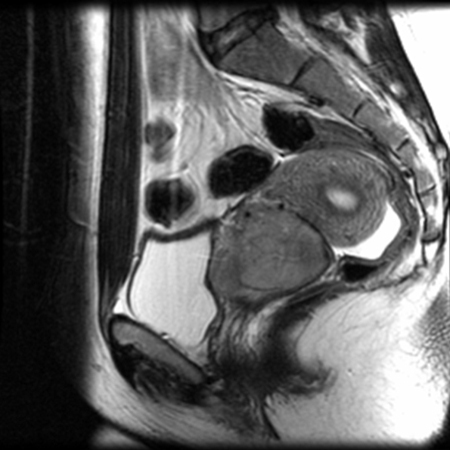
- MRI pelvis
- PET whole body
- PET/CT whole body
- CT of chest/abdomen/pelvis with intravenous/oral contrast
- molecular testing
Pruebas emergentes
- p16 and Ki67 biomarker expression
Algoritmo de tratamiento
non-pregnant stage IA1 without LVSI: desiring fertility
non-pregnant stage IA1 without LVSI: not desiring fertility
non-pregnant, stage IA1 with LVSI: desiring fertility
non-pregnant, stage IA1 with LVSI: not desiring fertility
non-pregnant stage IA2: desiring fertility
non-pregnant stage IA2: not desiring fertility
non-pregnant stage IB1: desiring fertility
non-pregnant stage IB1: not desiring fertility
non-pregnant stage IB2: desiring fertility
non-pregnant, stage IB2: not desiring fertility
non-pregnant stage IIA1
non-pregnant stage IB3 or IIA2
non-pregnant stage IIB to IVA
non-pregnant, stage IVB (metastatic disease)
non-pregnant local or regional recurrent disease
pregnant
Colaboradores
Autores
Richard T. Penson, MD, MRCP

Medical Gynecologic Oncologist
Division of Hematology Oncology
Massachusetts General Hospital
Boston
MA
Divulgaciones
RTP reports serving on scientific advisory boards for Aadi Bioscience, AstraZeneca, GSK Inc., ImmunoGen Inc., Merck & Co., Roche Pharma, Sutro Biopharma, Tubulis GmbH; and serves on or chairs data and safety monitoring boards for AstraZeneca, EQRx, and Roche Pharma. RTP receives institutional research funding (as Principal Investigator) from 858 Therapeutics; royalties from BMJ Publishing, UptoDate, Elsevier Ltd, Wolters Kluwer Health, and Wiley-Blackwell; and payment for educational events from Research to Practice, ExpertConnect, ReachMD, and CMEO Outfitters.
Andrea L. Russo, MD
Director
Gynecologic Radiation Oncology
Associate Clinical Director
Department of Radiation Oncology
Massachusetts General Hospital
Boston
MA
Раскрытие информации
ALR declares that she has no competing interests.
Выражение благодарностей
Dr Richard T. Penson and Dr Andrea L. Russo would like to gratefully acknowledge Dr Larissa J. Lee, their co-contributor who is sadly deceased, and to acknowledge Dr Neil S. Horowitz and Dr Anthony H. Russell, previous contributors to this topic.
Раскрытие информации
NSH and AHR declare that they have no competing interests.
Рецензенты
Tracilyn Hall, MD
Assistant Professor of Gynecologic Oncology
Dan L Duncan Comprehensive Cancer Center
Baylor College of Medicine Houston
Houston
TX
Раскрытие информации
TH declares that she has no competing interests.
Linda Yang, MD
Fellow
Minimally Invasive Gynecologic Surgery
Magee Women's Hospital
University of Pittsburgh Medical Center
PA
Раскрытие информации
LY declares that she has no competing interests.
Deirdre Lyons, MB, BCh, BAO, MRCOG
Consultant in Obstetrics & Gynaecology
Lead Clinician in Colposcopy
Imperial College Healthcare NHS Trust
London
UK
Раскрытие информации
DL declares that she has no competing interests.
Peer reviewer acknowledgements
BMJ Best Practice topics are updated on a rolling basis in line with developments in evidence and guidance. The peer reviewers listed here have reviewed the content at least once during the history of the topic.
Disclosures
Peer reviewer affiliations and disclosures pertain to the time of the review.
Список литературы
Основные статьи
Marth C, Landoni F, Mahner S, et al. Cervical cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017 Jul 1;28(suppl 4):iv72-83.Полный текст Аннотация
Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society CA Cancer J Clin. 2020 Sep;70(5):321-46.Полный текст Аннотация
Bhatla N, Aoki D, Sharma DN, et al. Cancer of the cervix uteri: 2021 update. Int J Gynaecol Obstet. 2021 Oct;155 Suppl 1(suppl 1):28-44.Полный текст Аннотация
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: cervical cancer [internet publication].Полный текст
Статьи, указанные как источники
A full list of sources referenced in this topic is available to users with access to all of BMJ Best Practice.
Отличия
- Human papillomavirus (HPV) infection
- Pelvic infection
- Nabothian cyst
Больше Отличияგაიდლაინები
- Suspected cancer: recognition and referral
- Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV
Больше გაიდლაინებიპაციენტის ბროშურები
Cervical cancer
HPV (human papillomavirus) vaccine
მეტი პაციენტის ბროშურებიშედით სისტემაში ან გამოიწერეთ BMJ Best Practice
ამ მასალის გამოყენება ექვემდებარება ჩვენს განცხადებას
